Artículos
Detection and isolation of Shiga toxin-producing Escherichia coli and microbial population counts on Argentinean Kosher beef for export to Israel
Detección y aislamiento de Escherichia coli productora de toxina Shiga y conteo de poblaciones microbianas en carne bovina Kosher argentina para exportar a Israel
ANALECTA VETERINARIA
Universidad Nacional de La Plata, Argentina
ISSN: 1514-2590
Periodicity: Frecuencia continua
vol. 43, e071, 2023
Received: 16 January 2023
Revised: 15 May 2023
Accepted: 14 June 2023
Abstract: The aim of this study was to provide data on the frequency of Top 7 Shiga toxin-producing Escherichia coli (STEC) and microbial population counts on processed beef from Argentinean Kosher cattle abattoirs authorized to export to Israel. A total of 480 samples (forequarters, primal cuts, trimmings) were taken and analyzed for Top 7 STEC detection and isolation and for mesophilic aerobic organism, coliform and E. coli enumeration. Differences in stx detection and microbial population counts on forequarter samples before and after salting were not statistically significant (P >0.05). All samples were negative for Top 7 STEC. Differences were significant for all microbial counts in primal cuts (P <0.001). Neck samples showed a higher level of contamination with the three groups of microorganisms than fore shank and brisket samples. The prevalence of stx was lower than that reported worldwide and in Argentinean export abattoirs. Salting did not significantly reduce the microbial load on forequarters.
Keywords: STEC, microbial counts, beef, Kosher, abattoir.
Resumen: El objetivo de este estudio fue determinar la frecuencia de Escherichia coli productora de toxina Shiga (STEC) Top 7 y realizar recuentos de poblaciones microbianas en carne Kosher de frigoríficos argentinos autorizados para exportar a Israel. Se tomaron 480 muestras (cuartos delanteros, cortes y recortes) y se realizó la detección y aislamiento de STEC Top 7 y el recuento de microorganismos aerobios mesófilos, coliformes y Escherichia coli. No se identificaron diferencias estadísticamente significativas (P > 0,05) en la detección de stx y en los recuentos de poblaciones microbianas en las muestras de cuartos delanteros antes y después del salado. Todas las muestras fueron negativas para STEC Top 7. Se hallaron diferencias estadísticamente significativas (P < 0,001) en todos los recuentos de poblaciones microbianas de los cortes. Las muestras de cogote tuvieron recuentos de poblaciones microbianas mayores a los de las muestras de brazuelo y pecho. La prevalencia de stx obtenida en el presente estudio fue menor a las reportadas en el resto del mundo y en frigoríficos exportadores de Argentina. El salado no redujo significativamente la carga microbiana de los cuartos delanteros bovinos.
Palabras clave: STEC, conteo microbiano, carne bovina, Kosher, frigorífico.
1. Introduction
Shiga toxin-producing Escherichia coli (STEC) are foodborne pathogens that can cause bloody diarrhea, hemorrhagic colitis, and hemolytic uremic syndrome (HUS). Incidence of HUS is lower in Israel than in most countries, especially because STEC-HUS is very rare (Alfandary et al., 2020).
STEC strains carried by cattle are unavoidably transferred to carcasses during slaughter (Duarte et al., 2016). Since beef can be contaminated at different stages from the abattoir to consumption (EFSA & ECDC, 2018), the current food safety preventive systems comply with the hazard analysis and critical control point (HACCP) principles (Sofos, 2008).
Slaughter performed under Jewish law is referred to as Kosher slaughter. It is performed by a specially trained person known as a “Shochet” and, in contrast to non-religious slaughter, the animal is not stunned prior to slaughter. The presence of defects is verified according to religious definitions (Farouk et al., 2014). If the carcass qualifies as Kosher, beef must go through the salting process to remove all the remaining blood (Zurek, et al., 2022). Only forequarters are intended for consumption.
Even though STEC frequency in Kosher beef production was unknown, in July 2019 the State of Israel implemented the zero-tolerance criteria for Top 7 STEC serogroups (O26, O45, O103, O111, O121, O145 and O157) on beef products.
The aim of this study was to provide data on the frequency of Top 7 STEC in processed beef from Argentine abattoirs authorized to export to Israel. In addition, microbial populations (total aerobic counts, E. coli and coliforms) were counted.
2. Materials and methods
2.1. Study design
The study was conducted between November 2020 and January 2021 in Argentinean Kosher cattle abattoirs authorized to export to Israel (IVSHA; SENASA). Twelve abattoirs complying with the current legal regulations for the religious practice of Kosher slaughter were invited and accepted to participate voluntarily in this study.
All abattoirs observed the following salting process (Akshará): 1) 30 min soaking (Ashraiá) of 1.5 l/kg of meat with cold (7 ºC) potable water using; 2) salting (Amlajá) applying coarse salt for 60 to 75 min; and 3) final washing (Akshrá) by immersing forequarters in three consecutive pools with cold (2 ºC) potable water. The entire process was monitored by each abattoir and verified by the National Service of Agrifood Health and Quality of Argentina (SENASA, for its Spanish acronym).
2.2. Sample collection
The following material was collected from each abattoir: 10 bovine forequarter samples before the salting process and 10 after 30-60 min salting; 18 Kosher primal cut samples before packaging (brisket n = 6; neck n = 6; fore shank n = 6); and 2 pools of trimmings (1kg each). From this material, a total of 480 samples were evaluated. They were distributed as follows: 60 right and 60 left bovine forequarter samples corresponding to the same animal before the salting process (n = 120); 60 right and 60 left bovine forequarter samples from the same animal after 30-60 min salting (n = 120); 216 Kosher primal cut samples before packaging (brisket n = 72; neck n = 72; fore shank n = 72); and 24 pools of trimmings (1-kg each) placed in sterile bags.
Two samples from each forequarter (before and after salting) and from each primal cut were used for microbial population counts (mesophilic aerobic organism, coliform and E. coli) and for Top 7 STEC search. For forequarter microbial population counts, a combination of two areas of 100 cm2 each (brisket and neck) was sponged. First, the brisket area was swabbed with one side of the sponge (ten strokes in two directions, from left to right and from the top to bottom) (Whirl-Pak Speci-Sponge, Nasco, US). The same sponge was then flipped to the other side to swab the neck area as described above. For primal cut microbial population counts, one area of 100 cm2 was sponged. For Top 7 STEC search, the total forequarter surface, including the external and internal surfaces, and the total primal cut surfaces were swabbed with another sponge. All sponges were sterile (Whirl-Pak) and had been previously soaked in 15 ml buffered peptone water (BPW, Biokar, Zac de Ther, France). After swabbing, sponges were placed into sterile stomacher bags. All sponges and trimming bags were stored at 4 °C and immediately sent to the “División Higiene y Seguridad Alimentaria y Ambiental, Stamboulian Servicios de Salud, Buenos Aires, Argentina”.
Sample size was enough to detect differences in stx prevalence of at least 16.0% (95.0% confidence level; 80.0% statistical power) on forequarters before and after the salting process. In primal cuts, sample size was sufficient to detect differences of at least 20% in stx prevalence (95.0% confidence level).
2.3. Microbiological analyses
2.3.1. Top 7 STEC detection and isolation
Ninety ml of BPW broth were added to each sponge and 400 ml of BPW broth was added to trimmings, following the ISO (2012) standard. Sponges were incubated for 24 h at 37 °C. The Top 7 STEC were detected using the bioMerieux Gene Up system (GENE-UP® STEC – TOP 6, GENE-UP® E. coli O157:H7 2, GENE-UP® STEC – stx & eae 2). If samples were positive for one of the serogroup-associated genes in the scope of the method, isolation was performed with immuno-concentration (VIDAS® UP E. coli serogroups) and streaked onto suitable medium.
2.3.2. Microbial population counts
Ninety ml of BPW broth were added to each sponge and mixed in the stomacher bag for 2 min. An aliquot (1 ml) was used for enumerations. Mesophilic aerobic organism (MAO) enumerations were performed plating in duplicate and incubating 72 h at 30 °C (ISO, 2013). Coliform enumerations were performed plating in duplicate and incubating 24 h at 37 °C (ISO, 2006). E. coli enumerations were performed plating in duplicate, using 5-bromo-4-chloro-3-indolyl-b-D-glucoronide and incubating 24 h at 44 °C (ISO, 2001).
2.4. Statistical analysis
Bacterial counts were log-transformed before statistical analyses. The effect of salting on forequarter stx detection and microbial population counts was assessed using a Generalized Linear Mixed Model (GLMM). Salting stages (before and after salting) were used as fixed effect. Forequarters nested to abattoir were the random term. Binomial distribution using a logarithmic link function and normal distribution were used for stx detection and microbial population counts, respectively. Variation on primal cut microbial population counts was described using GLMM with normal distribution, using type of primal cut as fixed effect and abattoir as random factor. All statistical analyses were performed using the Statistical Package for the Social Sciences Software (SPSS, IBM-SPSS, Inc., Chicago, IL, US), with a significance of P <0.05.
3. Results
3.1. Top 7 STEC detection and isolation
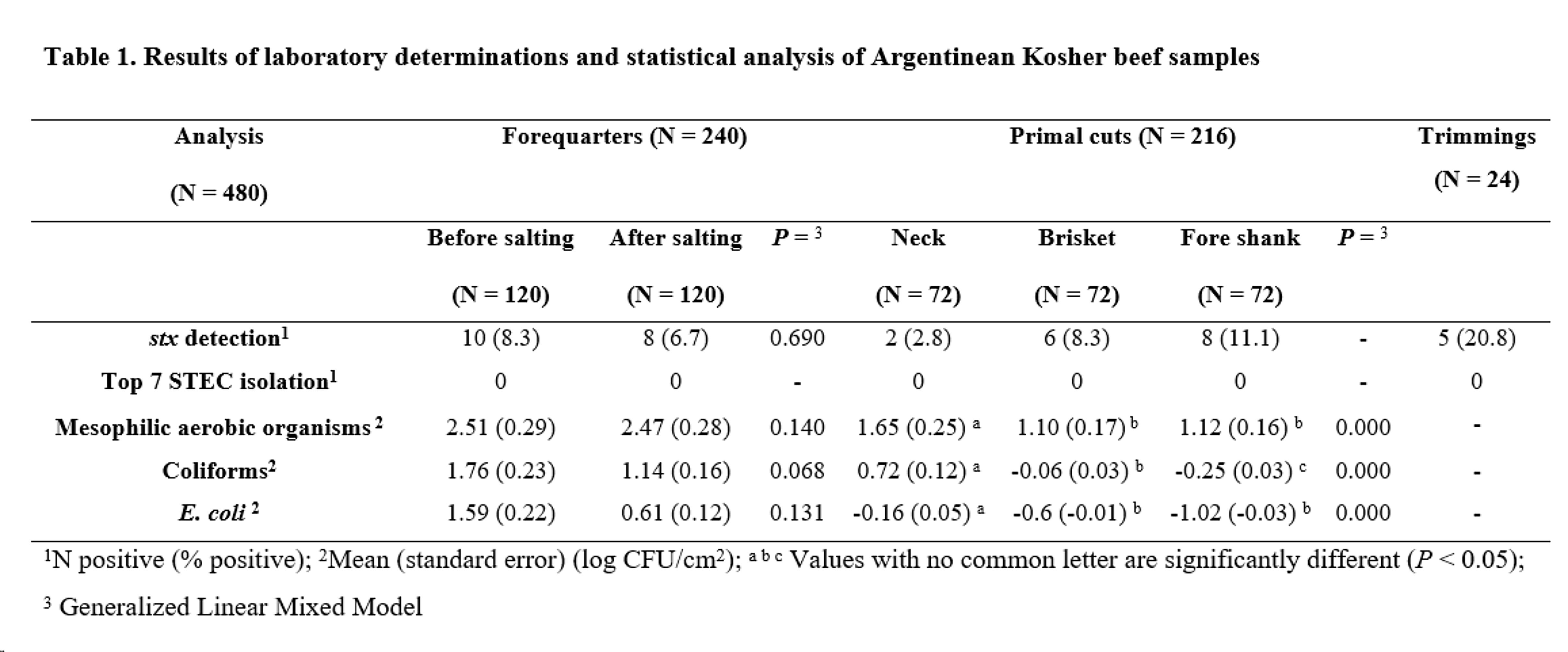
All samples were negative for Top 7 STEC serogroups. However, stx gene was detected in 8.3% of forequarters before salting, 6.7% of forequarters after salting, 2.8% of necks, 8.3% of briskets, 11.1% of fore shanks and 20.8% of trimmings. Differences in stx detection before and after forequarter salting were not statistically significant (P = 0.690) (Table 1).
3.2. Microbial population counts
The frequencies of samples with microbial population count on forequarters before and after salting were as follows: MAO, 104/120 and 106/120; coliforms, 51/120 and 46/120; E. coli, 31/120 and 27/120, respectively. Differences before and after salting were not significant (MAO P = 0.765; coliforms P = 0.068; E. coli P = 0.131). In the case of primal cuts, differences were significant for all microbial counts (P < 0.001). Neck samples showed a higher level of contamination with the three groups of microorganisms than fore shank and brisket samples. Additionally, brisket samples showed higher coliform counts than fore shank samples (Table 1).
4. Discussion
Scientific publications on the microbiology of Kosher beef are scarce worldwide, and none of them refer to STEC (Hajmeer et al., 2004; Zuckerman & Mannheim, 2001). For this reason, the results of the present study are discussed with reference to those of other authors who analyzed non-Kosher carcasses, primal cuts and trimmings.
In the present study, Top 7 STEC was not recovered from forequarters and primal cuts. The Top 7 STEC isolation rate on carcasses from the United States (US) (Barkocy-Gallagher et al., 2003; Cobbold et al. 2008; Stromberg et al., 2018) and Canada (Bohaychuk et al., 2011) was 0.09-57.0%. In Argentina, Masana et al. (2010; 2011) reported 7.02% of Top 7 STEC isolated from carcasses, and Brusa et al. (2017) isolated Top 7 STEC from 0.02% of primal cuts in Argentinean export abattoirs. Differences between studies could be attributed to variables such as cattle STEC load, and intervention measures and HACCP application at abattoir.
The stx detection rate on forequarters reported in this study was lower than described inthe European Union (EU) and the US , and similar to other reports from Argentina. In the EU and the US, stx detection was in the range of 13.4-60.6% on carcasses from abattoirs (Barkocy-Gallagher et al., 2003; Cobbold et al., 2008; Stromberg et al., 2018). In Argentina, previous studies have determined stx prevalence on carcasses from export abattoirs (Brusa et al., 2019, 2022; Signorini et al., 2018). In the present study, stx detection on forequarters before and after salting was similar to that reported by Brusa et al. (2019) (6,7%). In all forequarters, stx detection was higher than reported by Brusa et al. (2022) (3%), but lower than reported by Signorini et al. (2018) on carcasses (37.5%).
On primal cuts, stx was identified in 11-36% of samples from the EU (Nobili et al., 2017), the US (Cobbold et al., 2008) and Uruguay (Baeza Quiroz et al., 2013; Bosilevac et al., 2007). The mean stx detection from primal cuts reported in the present study (7.4%) was lower than the international rate and comparable to the 8.1% reported in Argentinean export abattoirs (Brusa et al., 2022).
The frequency of isolation of stx genes and E. coli O157:H7 in trimming samples from the US, Australia and New Zealand was 9.7-30 and 0.9-2.4%, respectively (Bosilevac et al., 2007; Carney et al., 2006). Detection frequency of stx gene in trimmings from the present study fell within the international range. In Argentina, Top 7 STEC were isolated in 0.03% of trimmings from export abattoirs (Brusa et al., 2017). In contrast with international and previous Argentinean publications, in the present study all trimming samples were negative for Top 7 STEC. These results are consistent with those informed by the Israel Veterinary Inspection Service (IVIS), whose analysis of 349 Argentine beef samples between July 2019 and December 2020 found that they were all negative for Top 7 STEC (IVIS, personal communication, March, 2021).
The effect of Kosher slaughter on beef microbial population counts has been previously evaluated at laboratory scale (Hajmeer et al., 2004; Zuckerman & Mannheim, 2001). Hajmeer et al., (2004) analyzed 10 Kosher briskets and 15 non-Kosher briskets, concluding that the salt applied during Koshering had a potential for microbial reduction and might prove beneficial for controlling E. coli O157:H7. The process had little success in reducing MAO, coliform and E. coli counts and the authors did not search for E. coli O157:H7. In another laboratory assay, eight non-Kosher primal cuts were processed with salt to emulate the Kosher treatment process (Zuckerman & Mannheum, 2001). The lower MAO counts found in salted as compared with unsalted primal cuts were explained by the effect of salt on the surface bacteria and by the effect of the massive soaking and rinsing involved in Koshering. Hajmeer et al. (2004) mentioned that an abattoir-based study cannot provide a valid scientific evaluation for line effectiveness of Koshering and the performance of laboratory scale studies. We consider that, despite the usefulness of laboratory scale studies , those carried out at abattoirs demonstrate the real effect of the treatment on the beef produced. The current results of 240 forequarter samples from export abattoirs applying all Kosher control measures suggest that the salting process did not significantly reduce either MAO, coliform and E. coli counts or stx detection.
It is interesting to consider that between 2010 to 2017, Argentinean export abattoirs raised concern to reduce the presence of STEC throughout the process of slaughter (HACCP-STEC) and marketing of beef products (Brusa 2017; Masana 2010, 2011). Improvement actions implemented at abattoir level, allowed to reduce, and even achieve zero STEC detection at Argentine abattoirs (Brusa 2017; 2019). In addition, in Argentina the effectiveness of applying interventions to reduce STEC was also evaluated (Brusa et al., 2019; Signorini et al., 2018), which would explain the absence of Top 7 STEC in this study. The current results provide evidence to predict low contamination with STEC and no contamination with the Top 7 STEC. However, it would be interesting to evaluate new interventions, such as high-pressure processing, ultrasound, radiation, ozone, organic acids, or other emerging processing techniques (Galli et al., 2016) that tend to reduce indicator microorganism counts in Kosher meat products, to extend shelf life.
5. Conclusions
Few studies on Kosher beef microbiology are available, and to our knowledge none exist on STEC. In this study, microbiological counts on Kosher beef were similar to those reported on non-Kosher beef and within the reference limits of international regulations. Although the prevalence of stx was lower than reported globally and in Argentinean export abattoirs, it was not possible to conclude that the absence of Top 7 STEC was due to the religious ritual process or the use of salt. Likewise, salting was not identified as a practice with significant effect to reduce the microbial load on forequarters. Other studies are necessary to evaluate the effect of Koshering on spoilage and pathogenic bacteria.
Acknowledgments
We thank A. Di Maggio for editing the manuscript. The authors disclosed receipt of the following financial support for the research, authorship, and/ or publication of this article: This study was supported by research grants from the Institute for the Promotion of Argentine Beef, IPCVA, R&D Program, under the Project “Characterization of risk and mitigation of impact of STEC in the beef chain.
Declaration of competing interest
Findings presented in this article are the interpretation from authors and do not necessarily represent the views of the funding agency. The authors declare they have no competing interest.
Author contributions
The authors contributed to this article as follows: Brusa,Victoria: Conceptualization, data curation, formal analysis, investigation, methodology, roles/writing - original draft, writing - review & editing. Blainq, Luis; Brasesco, Hebe; Bruzzone, Mariana; Buezas, Joaquín; Contardi, Ignacio; García, Diego; Gómez, Elda; Mariame, Mariela; Medici, Laura; Moretti, Georgina; Ochoa, Gonzalo; Petroli, Sandra and Superno, Valeria: Conceptualization & Methodology, Sucari, Adriana: Methodology. Vinelli, Francisco, and Albanese, Román: Conceptualization. Dolev, Sergio: Conceptualization, supervision, writing - review & editing. Signorini, Marcelo: Data curation, formal analysis, roles/writing - original draft. Leotta, Gerardo: Conceptualization, data curation, formal analysis, investigation, methodology, roles/writing - original draft, writing - review & editing, funding acquisition, project administration, resources, supervision.
References
Alfandary H, Rinatt C, Guerevich E, Eisenstein I, Golberg O, Kropach N, Landau D. 2020. Hemolytic uremic syndrome: A contemporary pediatric experience. Nephron. 144(3):109-17. https://doi.org/10.1159/000505401
Baeza Quiroz CB. 2013. Aislamiento y caracterización de cepas de Escherichia coli productor de shigatoxina desde carne de vacuno nacional e importada, distribuida en los principales supermercados de la provincia de Santiago. Trabajo de Tesis para optar al grado de Magíster en Salud Pública y Sistemas de Salud. Facultad de Medicina, Escuela de Salud pública, Universidad Mayor, Santiago, Chile. https://1library.co/document/ye9e2req-aislamiento-caracterizacion-escherichia-productor-shigatoxina-distribuida-principales-supermercados.html
Barkocy-Gallagher GA, Arthur TM, Rivera-Betancourt M, Nou X, Shackelford SD, Wheeler TL, Koohmaraie M. 2003. Seasonal prevalence of Shiga toxin producing Escherichia coli, Including O157:H7 and non-O157 serotypes, and Salmonella in commercial beef processing plants. Journal of Food Protection. 66(11):1978-86. https://doi.org/10.4315/0362-028X-66.11.1978
Bohaychuk VM, Gensler GE, Romero Barrios P. 2011. Microbiological baseline study of beef and pork carcasses from provincially inspected abattoirs in Alberta, Canada. Canadian Veterinary Journal. 52,1095-100.
Bosilevac JM, Guerini MN, Brichta-Harhay DM, Arthur TM, Koohmaraie M. 2007. Microbiological Characterization of imported and domestic boneless beef trim used for ground beef. Journal of Food Protection. 70(2):440-9. https://doi.org/10.4315/0362-028X-70.2.440
Brusa V, Restovich V, Galli L, Teitelbaum D, Signorini M, Brasesco H, Londero A, García D, Padola NL, Superno V, Sanz M, Petroli S, Costa M, Bruzzone M, Sucari A, Ferreghini M, Linares L, Suberbie G, Rodríguez HR, Leotta GA. 2017. Isolation and characterization of non-O157 Shiga toxin-producing Escherichia coli from beef carcasses, cuts and trimmings of abattoirs in Argentina. PLoS One. 12(8): e0183248. https://doi.org/10.1371/journal.pone.0183248
Brusa V, Restovich V, Signorini M, Pugin D, Galli L, Ruíz Díaz V, Arias R, Leotta GA. 2019. Evaluation of intervention measures at different stages of the production chain in Argentinian exporting abattoirs. Food Science and Technology International. 25(6):491-96. https://doi.org/10.1177/1082013219836326
Brusa V, Restovich V, Galli L, Arias R, Linares L, Costa M, Ruíz Díaz V, Pugin D, Leotta G. 2022. Reduction of Shiga toxin-producing Escherichia coli in beef abattoir. Food Science and Technology International.28(1):50-9. https://doi.org/10.1177/1082013221991258
Carney E, O'Brien SB, Sheridan JJ, McDowell DA, Blair IS, Duffy G. 2006. Prevalence and level of Escherichia coli O157 on beef trimmings, carcasses and boned head meat at a beef slaughter plant. Food Microbiology. 23(1):52-9. https://doi.org/10.1016/j.fm.2004.12.001
Cobbold RN, Davis MA, Rice DH, Szymanski M, Tarr PI, Besser TE, Hancook DD. 2008. Associations between bovine, human, and raw milk, and beef isolates of non-O157 Shiga toxigenic Escherichia coli within a restricted geographic area of the United States. Journal of Food Protection. 71,5:1023-7. https://doi.org/10.4315/0362-028X-71.5.1023
Duarte AS, Nauta M, Aabo S. 2016. Variation in the effect of carcass decontamination impacts the risk for consumers. Food Control. 59:12-9. https://doi.org/10.1016/j.foodcont.2015.05.015
EFSA & ECDC. 2018. The European Union summary report on trends and sources of zoonoses, zoonotic agents and food-borne outbreaks in 2017. EFSA Journal. 16(12):5500. https://doi.org/10.2903/j.efsa.2018.5500
Farouk MM, Al-Mazeedi HM, Sabow AB, Bekhit AED, Adeyemi KD, Sazili, AQ, Ghani A. 2014. Halal and kosher slaughter methods and meat quality: a review. Meat Science. 98 (3):505-19. https://doi.org/10.1016/j.meatsci.2014.05.021
Galli L, Brusa V, Rodríguez R, Signorini M, Oteiza JM, Leotta GA. Escherichia coli in food products. En: Torres A.G. (ed.). Escherichia coli in the Americas. Springer International Publishing AG, Cham, Switzerland, 2016.
Hajmeer MN, Marsden JL, Fung DYC, Kemp GK. 2004. Water, sodium chloride and acidified sodium chlorite effects on Escherichia coli O157:H7 and Staphylococcus aureus on beef briskets. Meat Science. 68:277-83. https://doi.org/10.1016/j.meatsci.2004.03.006
ISO/CEN 13136:2012. Microbiology of food and animal feed - Real-time polymerase chain reaction (PCR)-based method for the detection of food-borne pathogens - Horizontal method for the detection of Shiga toxin-producing Escherichia coli (STEC) and the determination of O157, O111, O26, O103 and O145 serogroups.
ISO 16649-2:2001. Microbiology of food and animal feeding stuffs - Horizontal method for the enumeration of beta-glucuronidase-positive Escherichia coli - Part 2: Colony-count technique at 44 degrees C using 5-bromo-4-chloro-3-indolyl beta-D-glucuronide.
ISO 4832:2006(E). Microbiology of food and animal feeding stuffs - Horizontal method for the enumeration of coliforms - Colony-count technique.
ISO 4833-1:2013. Microbiology of the food chain - Horizontal method for the enumeration of microorganisms - Part 1: Colony count at 30 °C by the pour plate technique.
Masana MO, Leotta GA, Del Castillo LL, D´Astek BA, Palladino PM., Galli L, Vilacoba E, Carbonari C, Rodríguez HR, Rivas M. 2010. Prevalence, characterization, and genotypic analysis of Escherichia coli O157:H7/NM from selected beef exporting abattoirs of Argentina. Journal of Food Protection. 7(4):649-56. https://doi.org/10.4315/0362-028X-73.4.649
Masana MO, D´Astek BA, Palladino PM, Galli L, Del Castillo LL, Carbonari LL, Leotta GA, Vilacoba E, Irino K, Rivas M. 2011. Genotypic characterization of non-O157 Shiga toxin–producing Escherichia coli in beef abattoirs of Argentina. Journal of Food Protection. 74(12):2008-17. https://doi.org/10.4315/0362-028X.JFP-11-189
Nobili G, Franconieri I, La Bella G, Basanisi MG, La Salandra G. 2017. Prevalence of Verocytotoxigenic Escherichia coli strains isolated from raw beef in southern Italy. International Journal of Food Microbiology. 257:201-5. https://doi.org/10.1016/j.ijfoodmicro.2017.06.022
Servicio Nacional de Sanidad y Calidad Agroalimentaria (SENASA). 2014. Resolución N° 247/2014. Retrieved from https://www.argentina.gob.ar/normativa/nacional/resoluci%C3%B3n-247-2014-230711/texto.
Signorini M, Costa M, Teitelbaum D, Restovich V, Brasesco H, García D, Superno V, Petroli S, Bruzzone M, Arduini V, Vanzini M, Sucari A, Suberbie G, Turina M, Rodríguez R, Leotta GA. 2018. Evaluation of decontamination efficacy of commonly used antimicrobial interventions for beef carcasses against Shiga toxin-producing Escherichia coli. Meat Science. 142:44-51. https://doi.org/10.1016/j.meatsci.2018.04.009
Sofos JN. 2008. Challenges to meat safety in the 21st century. Meat Science. 78(1-2):3-13. https://doi.org/10.1016/j.meatsci.2007.07.027
Stromberg ZR, Redweik GAJ, Mellata M. 2018. Detection, prevalence, and pathogenicity of non-O157 Shiga toxin-producing Escherichia coli from cattle hides and carcasses. Foodborne Pathogens and Disease. 15(3):119-31. https://doi.org/10.1089/fpd.2017.2401
Zurek J, Rudy M, Duma-Kocan P, Stanisławczyk R, Gil M. 2022. Impact of Kosher slaughter methods of heifers and young bulls on physical and chemical properties of their meat. Foods. 11:662. https://doi.org/10.3390/foods11040622
Zuckerman H & Mannheim CH. 2001. Color improvement of Kosher beef using sodium ascorbate and erythorbate. Journal of Muscle Foods. 12:137-51. https://doi.org/10.1111/j.1745-4573.2001.tb00305.x
