Trabajos de investigación
Insulin-like growth factors in cat placenta
Factores de crecimiento similares a insulina en la placenta de la gata
Analecta Veterinaria
Universidad Nacional de La Plata, Argentina
ISSN: 0365-5148
ISSN-e: 1514-2590
Periodicity: Frecuencia continua
vol. 44, e090, 2024
Received: 11 July 2024
Revised: 28 October 2024
Accepted: 31 October 2024
Corresponding author: rhernandez@fcv.unlp.edu.ar

This work is licensed under Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International.
Abstract: Placental development involves maternal-fetal signalling events, and it is an under-researched topic in feline reproduction. Few studies of endotheliochorial placentas deal with insulin-like growth factors (IGFs), which are among the principal developmental regulators. While placental expression of IGFs and type 1 IGF receptor (IGF1R) has already been reported in bitches, data regarding the IGF system in queens are limited to uterine tissues. This study aimed to detect IGF1, IGF2, and IGF1R in fetal and maternal placental structures. Samples from twenty-three placentas were classified into one of two groups depending on gestational age (G1: ≤43 d.p.c; G2: ≥44 d.p.c) and processed by indirect immunohistochemistry. Labelling with all the antibodies was stronger in the endometrial glands of earlier placentas than in later ones. Maternal endothelium was moderate to strongly labelled, with a decreasing intensity in the endometrium, while the opposite occurred in the labyrinth. Cytotrophoblast cells were more strongly labelled than the syncytiotrophoblast. IGF1 and IGF1R-positive cells were more abundant in decidual cells of later placentas. These results support that the IGF system plays a central role during gestation and development in cats. As far as we know, this is the first report recording immunohistochemical IGFs/IGF1R detection in fetal regions of the feline placenta.
Keywords: cat, placenta, insulin-like growth factors, trophoblastic cells, immunohistochemistry.
Resumen: La señalización materno-fetal durante el desarrollo placentario es un tema poco investigado en reproducción felina. Los factores de crecimiento similares a insulina (IGF) son importantes reguladores del desarrollo, escasamente estudiados en placentas endoteliocoriales. La expresión placentaria de IGFs y del receptor IGF tipo 1 (IGFR1) ya se ha descrito en perras; en gatas solo se han descrito en el útero. El objetivo de este estudio fue detectar IGF1, IGF2 e IGFR1 en estructuras placentarias fetales y maternas. Veintitrés muestras de placentas fueron clasificadas en dos grupos, según la edad gestacional (G1: ≤43 d.p.c; G2: ≥44 d.p.c); las mismas se procesaron mediante inmunohistoquímica indirecta. La marcación con todos los anticuerpos fue más intensa en las glándulas endometriales de placentas tempranas que en tardías. El endotelio materno se marcó de manera moderada a fuerte, con intensidad decreciente hacia los vasos del endometrio. Las células del citotrofoblasto se marcaron más que el sincitiotrofoblasto. IGF1 e IGF1R fueron más abundantes en las células deciduales de las placentas tardías. Estos resultados permiten sostener la centralidad del sistema IGF durante el desarrollo placentario felino. Según nuestro conocimiento, este es el primer trabajo que informa y describe la detección inmunohistoquímica de IGFs/IGFR1 en regiones fetales de la placenta felina.
Palabras clave: gata, placenta, factores de crecimiento similares a la insulina, células trofoblásticas, inmunohistoquímica.
1. Introduction
Placental development is a complex process involving signalling events between fetal and maternal cells, yet mechanisms underlying these phenomena during feline placentogenesis have not been studied in depth. Like most carnivores, cats develop a chorioallantoic, belt-shaped placenta with an endotheliochorial interface (Wooding & Burton, 2008). The main component of the placental girdle is the labyrinth, which comprises a body and a front that is included in the maternal-fetal junctional zone. The paraplacenta is formed by the hematophagous organs (less evident than in dogs), which may border the girdle by the free polar zone and the interfetal polar zone. The free polar zone is composed of columnar trophoblastic cells facing the endometrial epithelium. In the body of the labyrinth, maternal components (blood vessels and decidual cells) and fetal ones (blood vessels in the mesenchymal villous core and trophoblastic cells) are arranged to form maternal and fetal parallel lamellae (Leiser & Koob, 1993).
A significant part of the maternal-fetal dialogue is established between decidual cells (DCs) and trophoblastic cells. DCs are the only non-vascular maternal cells that remain in the labyrinth after epithelial degeneration. They are located between maternal vessels and the syncytiotrophoblast (Leiser & Koob, 1993). They have several functions and have been thoroughly studied in experimental animals and humans. The invasion of maternal tissues by trophoblastic cells is modulated by DCs through the balanced secretion of pro- and anti-invasive molecules, leading to a timely and controlled invasion (Sharma et al., 2016). The trophoblast of the cat girdle includes the cytotrophoblast (CTB) and the syncytiotrophoblast (STB). Cytotrophoblast is composed of discrete cells overlaying the mesenchyme. Syncytiotrophoblast is formed as a result of CTB cell fusion and differentiation (Huppertz, 2010; Leiser & Koob, 1993).
Insulin-like growth factors (IGFs) are among the main placental development regulators; they control the placental size, morphology, and functions, consequently influencing fetal growth. The IGF system includes IGF1, IGF2, and insulin, their tyrosine kinase receptors IGF1R, IGF2R, and the insulin receptor, six binding proteins (IGFBPs), and their proteases. Insulin-like growth factors are evolutionarily conserved peptides that induce cell proliferation, protein synthesis, survival, migration, and differentiation in many cell types, mainly by binding to IGF1R. Insulin-like growth factors availability, and thus biological activities, are modulated by IGFBPs, which have a higher affinity for IGFs than IGF1R. Insulin-like growth factors binding proteins may be anchored to the plasma membrane or extracellular matrix elements, accentuating IGFs concentration in the nearby environment of the cells. The downstream IGF1R signalling pathway includes the activation of several intracellular signalling molecules, such as phosphoinositide3-kinase/protein kinase B/mammalian target of rapamycin (LeRoith et al., 2021).
Current knowledge about the expression and functions of the IGF system in placental organogenesis comes mainly from studies dealing with hemochorial placentas. Both in vivo and in vitro studies in those placentas have shown that IGFs exert endocrine, autocrine, and paracrine actions in regulating placental functions and fetal growth (Roberts et al., 2008; Sferruzzi-Perri et al., 2011). Recently, the association between disturbed trophoblastic IGF signalling and fetal growth idiopathic restriction has been demonstrated in human placentas. In humans, the dysregulation of IGF signalling might lead to an alteration of trophoblastic cell turnover, with an increment in the apoptosis rate (Harris et al., 2019). Besides, the IGF system participates in myometrial phenotypic and functional changes during gestation, including an initial proliferative phenotype and a subsequent synthetic phase resulting in myocyte hypertrophy (Shynlova et al., 2009). In ewes, which develop a less invasive (synepitheliochorial) placenta, IGF2 mRNA transcripts were demonstrated in maternal cells and chorioallantoic mesenchyme, but not in TB cells (Igwebuike, 2010). In the epitheliochorial placenta of the mare, only TB cells from the endometrial cups (the most invasive TB population) were shown to produce IGF2 (Lennard et al., 1995).
Insulin-like growth factor 1 is paramount to placental growth and development by regulating trophoblast differentiation, proliferation, and survival (Forbes & Westwood, 2008a). Insulin-like growth factor 2 activity has been related to the increasing volume of the placental labyrinth, based mainly on angiogenesis, trophoblastic cell proliferation, and STB formation (Herr et al., 2009; Sferruzzi-Perri, 2018). It has been suggested that IGF2 plays a role in adapting placental transport capacity during development (Sferruzzi-Perri, 2018) and stimulates trophoblast invasion of maternal tissues (Forbes & Westwood, 2008a). The mitogenic, pro-survival, and metabolic actions of IGF2 are thought to be mediated mainly through IGF1R (Sferruzzi-Perri et al., 2017).
A few studies deal with the expression of IGFs/IGFRs in endotheliochorial placentas. In bitches, Kautz described that IGF2 and IGF1R are found in the endometrial glands and the myometrium, even before embryo implantation occurs (Kautz et al., 2014). As we reported previously (Hernández et al., 2020), endometrium, myometrium, and cell populations of the canine placenta (including trophoblastic cells) express IGFs/IGF1R at least in some stages of gestation. Trophoblastic IGF2 transcription was reported in the elephant endotheliochorial placenta (Wooding et al., 2005). Ağaoğlu et al. (2016) reported significant differences in IGF2 mRNA concentration between pre- and post-implantation samples of the queen’s uterus. More recently, the same research group detected IGFs and IGFRs mRNA and the corresponding proteins from pre-implantation to mid-gestation in uterine tissues (Ağaoğlu et al., 2021).
Numerous studies concerning the IGF system have been conducted in pregnant uterus and placentas, mainly in hemochorial ones; three of them concern the feline uterus (Ağaoğlu et al., 2021; Boomsma et al., 1994). However, to our knowledge, there are no available reports about the IGF system in specific cell populations of the feline placenta. This study aimed to detect IGF1, IGF2, and IGF1R in the cat placenta and to pinpoint the location of these proteins in particular fetal and maternal placental structures.
2. Methods
2.1. Placental samples
Samples from 23 mixed-breed queens were obtained by veterinarians after owner-required and authorised ovary hysterectomies. Although most females were going through their first heat, precise data on the queen’s age was not available. Samples were formalin-fixed for 24 h and processed using routine histological techniques. Three-micrometer sections were either stained with haematoxylin and eosin or mounted on positively charged slides for further immunohistochemical procedures (Biotraza microscope slides, Cat. #HDA S001A, Huida Medical Instruments Co., Jiangsu, China).
The determination of gestational age was based on the general external characteristics of each embryo-fetus, including the crown-rump length and several developmental features (Evans & Sack, 1973; Knospe, 2002; Pieri et al., 2015). Then, each sample was assigned to one of two groups: group 1 (G1., which included samples from ≤43 days post coitum (d.p.c) placentas, or group 2 (G2), in which ≥44 d.p.c to term placental samples were included. The 44 gestational day corresponds to the beginning of stage 20 of prenatal development in the cat (Knospe, 2002). Placentas were classified in that way to analyse the general tendency in IGF1, IGF2, and IGF1R expression. Fourteen samples were assigned to G1; the remaining 9, belonging to 44 to 55 d.p.c, were included in G2.
2.2. Antibodies
The following antibodies were used: anti-IGF1 (1/600, polyclonal, Biorbyt, orb101730, Saint Luis, USA), anti-IGF2 (1/500, polyclonal, Biorbyt, orb10887, Saint Luis, USA) and anti-IGF1R (1/200, Santa Cruz Biotechnology, sc-712, Dallas, USA). Besides, considering that IGFs/IGF1R and their products have high phylogenetic conservation (Cherif-Feildel et al., 2019; Rentería et al., 2008), we conducted a similarity search using BLAST (http://www.ncbi.nlm.nih.gov/BLAST) to infer the homology between target protein sequences of each anti-human antibody (Homo sapiens) and the homologous protein of cats. The sequence similarity percentages were: 97.01% for IGF1, 89.19% for IGF2, and 98.10% for IGF1R. This analysis enables further identification of protein variants and regions of the proteins that are highly conserved in orthologous species (Roncador et al., 2015). This search tool was used to identify the peptides that function as antigens and thus are recognised by the antibodies (Liu et al., 2013). Corroboration of the similarity between molecules from other species and from humans using BLAST has been conducted and previously reported by Baravalle et al. (2015), Sferruzzi-Perri et al. (2017), and Stassi et al. (2019). IGF1, IGF2, and IGF1R are homologous and orthologous conserved genes in mammals; between Homo sapiens and Felis catus, large similarity percentages in nucleotide sequences were confirmed.
2.3. Immunohistochemistry
Sections for immunohistochemical analysis were dewaxed in xylene, rehydrated through a graded series of ethanol, and washed in PBS (2 × 5 min each). Endogenous peroxidase activity was blocked with 1% hydrogen peroxide in methanol for 30 min at room temperature and washed with PBS (2 × 5 min each). Slides were subsequently immersed in citrate buffer (0.01M, pH 6.0) for antigen retrieval, which was performed using microwave irradiation (800W). Samples were irradiated for 3 min at 100% power, then for 6 min at 40% power, and subsequently washed with PBS (2 × 5 min each). Non-specific bindings were blocked with 10% goat non-immune serum (Sigma-Aldrich, Missouri, USA) for 30 min at room temperature. Overnight incubation with primary antibodies was performed at 4°C. Adjacent sections were subjected to the same immunohistochemical method, replacing primary antibodies with rabbit non-immune serum to verify immunoreaction specificity. After washing with PBS (2 × 5 min), samples were subsequently incubated with the universal secondary antibody using the streptavidin-peroxidase method (CytoScan™HRP Detection System, Cell Marque™, Rocklin, USA) for 30 min at room temperature. Liquid 3,3-diaminobenzidine tetrahydrochloride (DAB) was used as the chromogen (Liquid DAB-Plus Substrate Kit; Cell Marque™, Rocklin, USA), for 3 min 10 sec (IGF1), 3 min (IGF2) or 1 min 30 sec (IGF1R). Some sections were incubated with DAB alone to exclude the possibility of non-suppressed endogenous peroxidase activity. Slices were processed by omitting the primary antibodies for IGF1, IGF2, and IGF1R, as negative controls. The golden-brown DAB-H.O. reaction product was indicative of positively stained structures. Mayer’s haematoxylin was used as a nuclear counterstain (Biopur, Rosario, Argentina). Slides were observed using an Olympus BX53 microscope (Olympus, Tokyo, Japan), and representative images were taken with a digital camera (DP73, Olympus) in TIFF format. IGFs and IGF1R localization and distribution in the different placental zones were analysed.
2.4. Analysed zones and structures
In the region of the placental girdle, three zones were analysed: the maternal zone, the junctional zone, and the labyrinth (Figure 1). Besides, the results of the paraplacental free polar zone epithelia were described. The labelling intensity of connective tissue, endometrial glands, endothelium, vascular smooth muscle, and myometrium was assessed in the maternal zone. As components of the “junctional zone” (JZ) were considered: the junctional CTB (JZ-CTB), the junctional STB (JZ-STB), both forming the front of the labyrinth and the histotroph (Figure 1B3). As the “labyrinth,” both the body of the lamellar labyrinth and the fetal zone were described. Thus, we recorded the labelling intensity of fetal endothelia (from chorioallantoic vessels), mesenchymal cells, CTB, STB, DCs, and maternal endothelia (Figures 1B1, B2). Regarding the paraplacenta, trophoblast cells, and the opposite uterine epithelium were evaluated. The qualitative assessment of staining intensity was performed blindly by two independent evaluators. Staining intensity was categorised as being (+), (++), or (+++) if it was mild, moderate, or strong, respectively. This scale was determined for each antibody separately. The absence of labelling was recorded as (-), and the inconsistent labelling as (±).
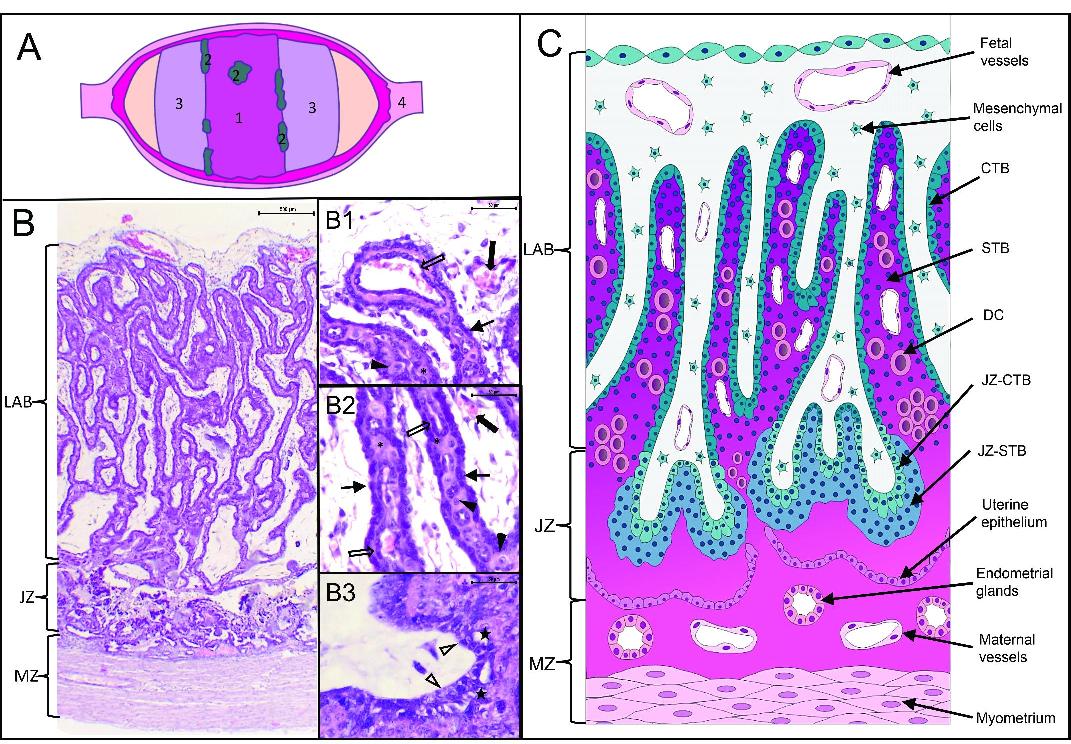
Figure 1
Feline placenta. (A) Schematic diagram of the placental gross appearance (cut surface of the uterus).1. Placental girdle; 2. Hematophagous organs; 3. Free polar zones; 4. Interplacental uterine site. (B) Structure of the feline placenta. Hematoxylin and eosin (HE). Laminar arrangement of maternal and fetal structures (Bar=500 μm). (B1, B2, B3) magnification of B. (B1) Fetal zone (Bar=50 μm). (B2) Labyrinth (Bar=50 μm). (B3) Junctional zone (Bar=50 μm). LAB: labyrinth; JZ: junctional zone; MZ: maternal zone; thin arrows: cytotrophoblast (CTB); asterisks: syncytiotrophoblast (STB); black arrowheads: decidual cells (DC); thick empty arrows: maternal endothelium; thick black arrows: fetal vessels; empty arrowheads: JZ-CTB; black stars: JZ-STB. (C) Schematic representation of the placental girdle showed in (B).
In addition, for an overall evaluation, a quantitative analysis of five random placental images (per case) was conducted. Images were taken from the histological sections using an Olympus BX53 microscope (Olympus, Tokyo, Japan) and an Olympus DP73 digital camera (Olympus, Tokyo, Japan). Data from each tissue were analysed and expressed as a percentage of the immunostained area (Diessler et al., 2007), dismissing any eventual non-specific staining. Briefly, the percentage of the immunostained area was calculated using the following formula:
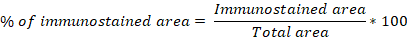
where, Immunostained area corresponded to the calibrated pixels representing the tissue areas being positive for the immunohistochemical reaction, and Total area was the calibrated area of the pixels representing the entire resolution of the image. The Immunostained area was determined using the image analysis software Image Pro Plus (v3.6 – Media Cybernetics, USA).
2.5. Statistical analysis
The data are presented as mean ± SEM. Mean values were obtained for comparison between two groups using unpaired Student’s t test or analysis of variance, followed by the Student–Newman–Keuls (SNK) test for multiple comparisons using Graph Pad Prism 8.0.2 software. For all analyses, the differences were considered significant when P < 0.05 (Santos et al., 2021).
2.6. Registration and ethics committee
The Institutional Committee for the Care and Use of Laboratory Animals (CICUAL, Veterinary School, National University of La Plata) approved all the procedures (protocol number Nº 58-3-16T).
3. Results
3.1. Immunohistochemistry
The results for IGF1, IGF2, and IGF1R staining patterns are summarized in Table 1. Briefly, almost all tissue structures showed labelling of both growth factors (GFs) and IGF1R. No staining was observed in slides used as negative controls (Figures 2A, B, C).
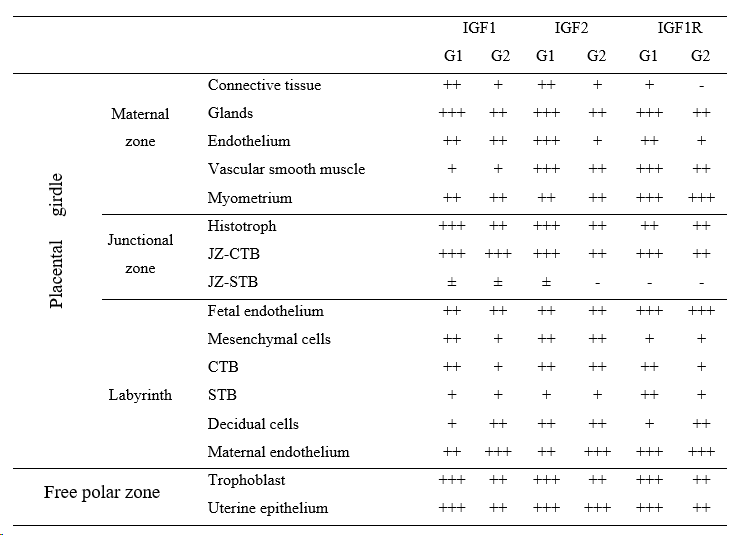
Abbreviations CTB: cytotrophoblast; G1: group 1; G2: group 2; JZ: junctional zone; STB: syncytiotrophoblast. Labelling intensity: (-): no labelling; (±): inconsistent; (+): mild; (++): moderate; (+++): strong.
Figure 2
Negative controls for the immunohistochemical detection of (A) IGF1R, (B) IGF1, and (C) IGF2 in the feline placenta. IHC. DAB. (Bar=500 μm).
Most of the components in the maternal zone, but especially the endometrial glands, were positive for IGF1, IGF2, and IGF1R in samples from both groups. The labelling ranged from moderate to strong for the three proteins. Besides, it was distinctly stronger in G1 than in G2, except for the myometrium. That change was observed in the histotroph but only for IGFs (Figures 3A, B, C, D, E). The feline myometrium in different stages of pregnancy was positive for IGF1, 2, and IGF1R, and remained relatively constant (Figure 3F). Maternal endothelium was moderate to strongly labelled, with a general tendency to a decreasing intensity in the endometrial vessels and increased intensity in the labyrinthine ones, from G1 to G2 placentas (Figures 3A, B, C, D; Figure 4).
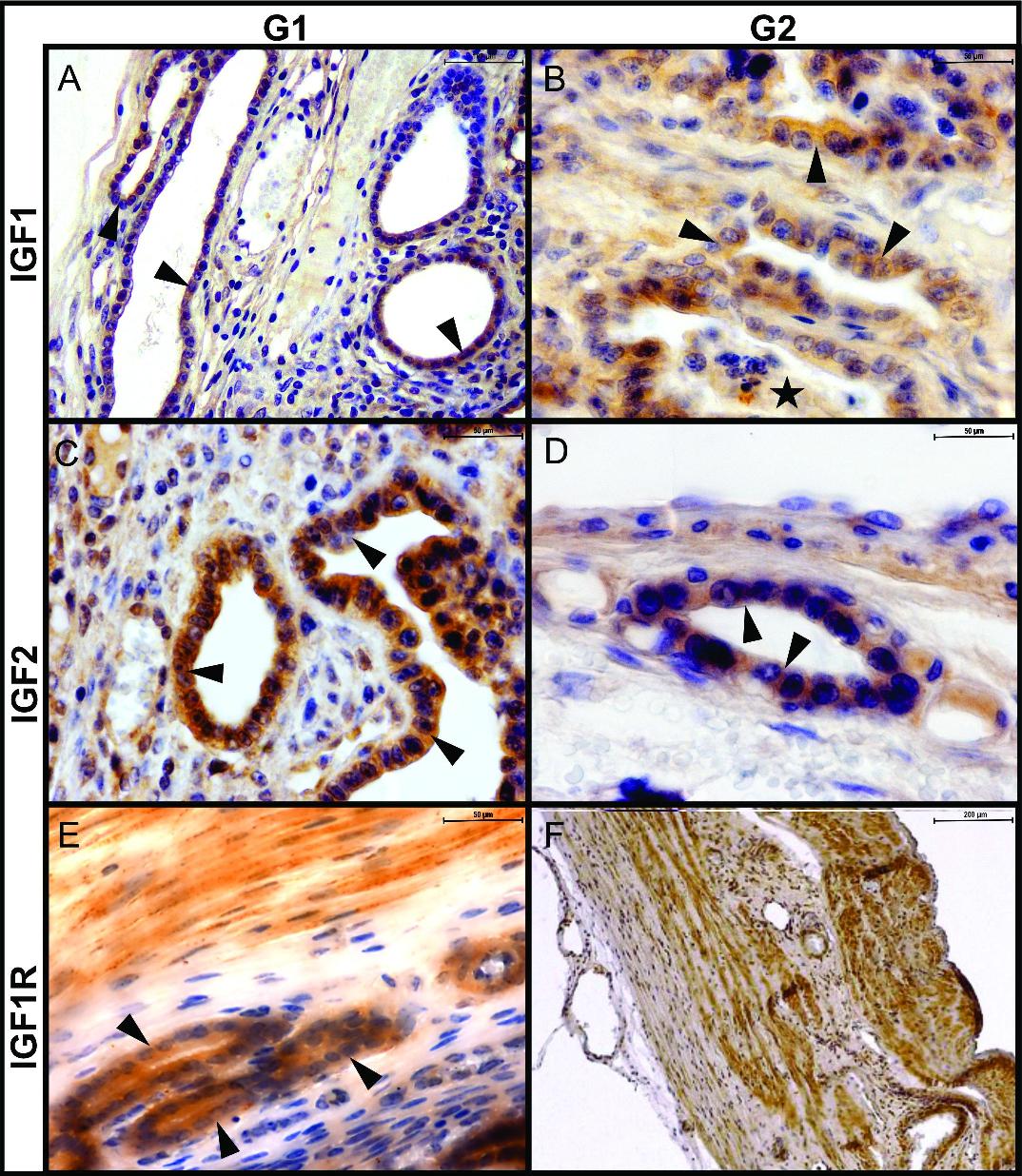
Figure 3
Feline placenta. Immunohistochemical detection of IGF1, IGF2, and IGF1R in the maternal zone, in samples from groups 1 (G1) and 2 (G2). Cytoplasmic labelling of the three molecules (A) IGF1, G1. Strong labelling in glandular cells (arrowheads (Bar=100μm). (B) IGF1, G2. Histotroph (stars) and glandular cells are moderately labeled (Bar=50μm). (C) IGF2, G1. Strong labelling in glandular cells (Bar=50 μm). (D) IGF2, G2. Moderate labelling in glandular cells (Bar=50 μm). (E) IGF1R, G1. Strong labelling in glandular cells (Bar=50 μm). (F) IGF1R, G2. Strong labelling in myometrial myocytes (Bar=200μm). Arrowheads: glandular cells; stars: histotroph. IHC. DAB.
As to the labyrinth, trophoblastic cells were positive for IGFs and IGF1R. The CTB showed stronger labelling of IGFs than the STB, both in the labyrinth and the junctional zone; JZ-STB labelling was inconsistent for the GFs and negative for the receptor (Figure 4; Figures 5A, B, C, D). Signals of IGF1 and IGF1R decreased in the labyrinthine CTB in samples of G2, while IGF2’s remained unchanged (Figure 4). Maternal and fetal endothelia in the labyrinth were positive for IGFs and IGF1R (with the abovementioned increase in maternal ones). Concerning decidual cells, IGF1 and IGF1R were more abundant in decidual cells of G2 placentas, whereas IGF2 was equally labelled in DCs from both groups (Figure 4).
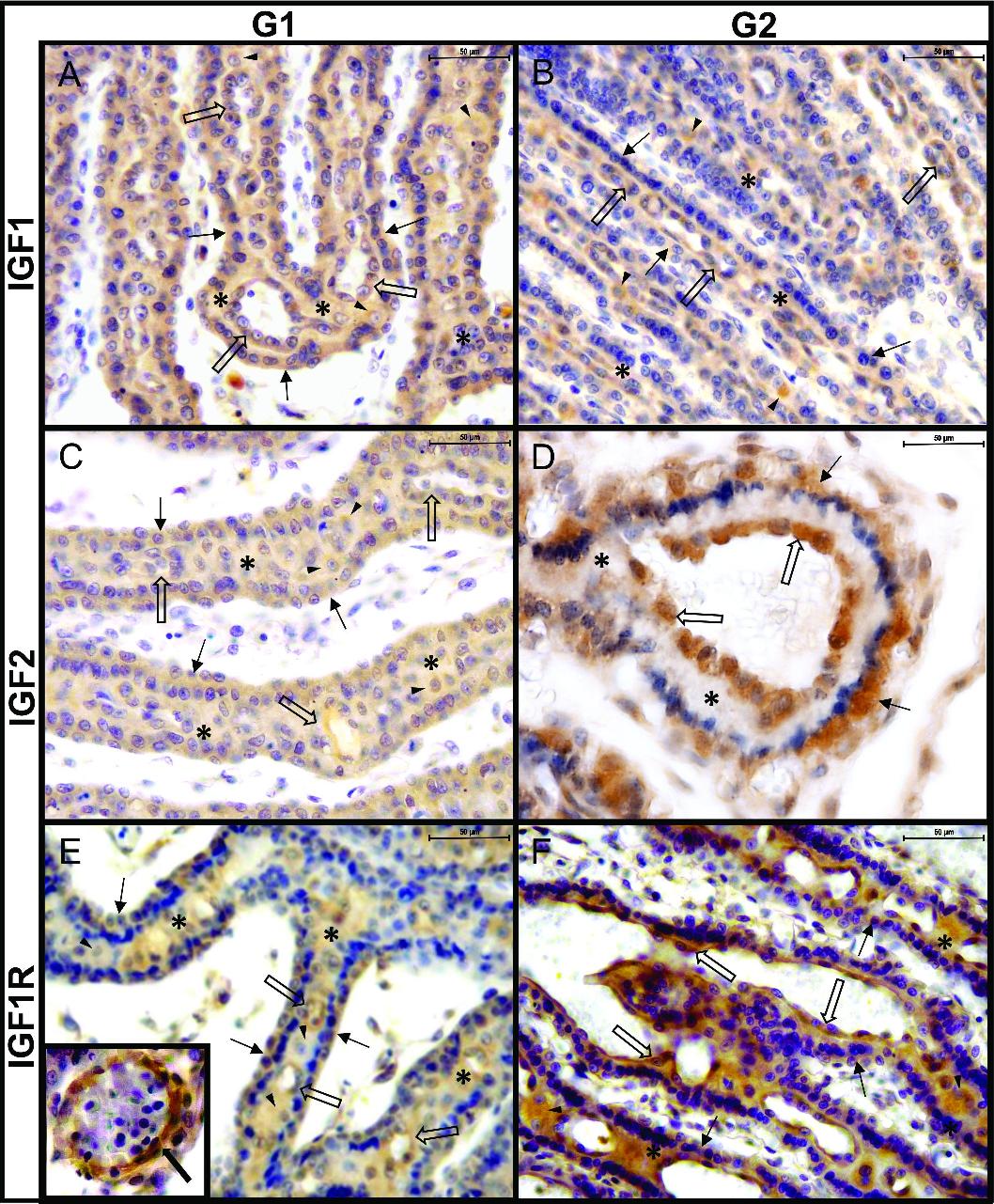
Figure 4
Feline placenta. Immunohistochemical detection ofIGF1, IGF2, and IGF1R in the labyrinth, in samples from groups 1 (G1) and 2 (G2). (A) IGF1, G1. Moderate labelling in CTB (thin arrows) and maternal endothelium (thick empty arrows). DC (arrowheads) and STB (asterisks) were mildly labelled. (B) IGF1, G2. Mild labelling in CTB and STB, moderate in DC, and strong in the maternal endothelium. (C) IGF2, G1. Moderate labelling in CTB, maternal endothelium, and DC. Mild labelling in STB. (D) IGF2, G2. Moderate labelling in CTB, mild labelling in STB. Maternal endothelium is strongly labelled. (E) IGF1R, G1. Moderate labelling in CTB and STB. Mild labelling in DCs and strong in the maternal endothelium. Inset: strong labelling in the endothelium of a fetal vessel. (F) IGF1R, G2. Mild labelling in CTB and STB. The DCs are moderately labelled. Strong labelling in maternal endothelium. Thin arrows: cytotrophoblast (CTB); black arrowheads: decidual cells (DC); asterisks: syncytiotrophoblast (STB); thick empty arrows: maternal endothelium; thick black arrow: fetal vessel. IHC. DAB. (Bar=50 μm).
Concerning the free polar zone, both epithelia were positive for IGF1, IGF2, and IGF1R. While IGF1 and IGF1R decreased in endometrial lining epithelium from samples in G1 to those in G2, IGF2 remained constant (Figures 5E, F).
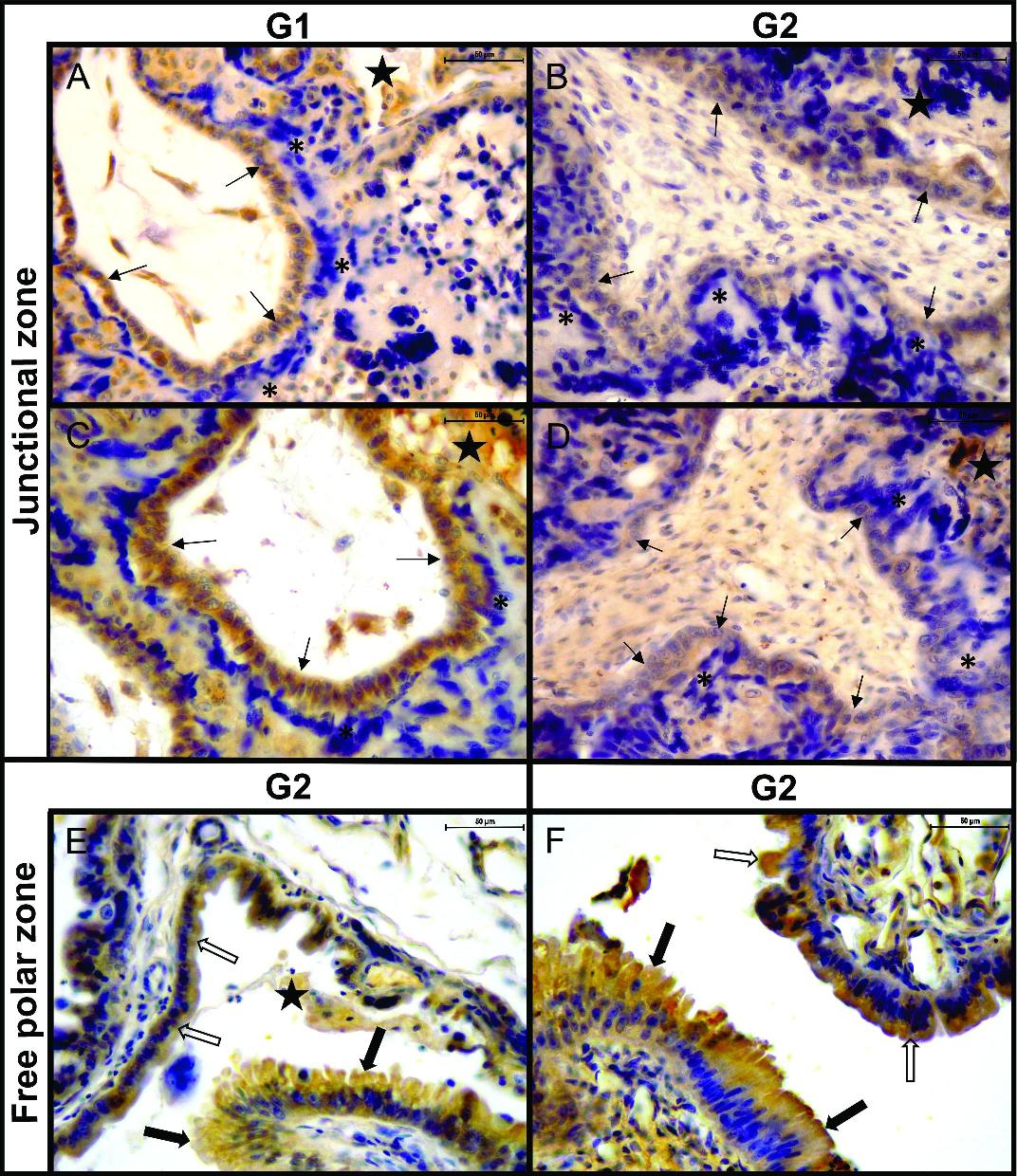
Figure 5
Feline placenta. Immunohistochemical detection of IGF1, IGF2, and IGF1R in the junctional zone (JZ) and the free polar zone. (A) JZ. IGF1, G1. Strong labelling in the JZ-CTB (thin arrows) and the histotroph (black stars). The labelling in the JZ-STB (asterisks)was inconsistent (negative in this image). (B) JZ. IGF2, G2. Moderate labelling in JZ-CTB and histotroph. The JZ-STB was negative. (C) JZ. IGF1R, G1. Strong labelling in JZ-CTB and histotroph. The JZ-STB was negative. (D) JZ. IGF1R, G2. Moderate labelling in JZ-CTB and histotroph. The JZ-STB was negative. (E) Free polar zone. IGF1, G2. Moderate labelling in the uterine epithelium (thick empty arrows), the trophoblast (thick black arrows), and the histotroph. (F) Free polar zone. IGF1R, G2. Moderate labelling in the uterine epithelium and the trophoblast. Thin arrows: cytotrophoblast of junctional zone (JZ-CTB); asterisks: syncytiotrophoblast of junctional zone (JZ-STB); black stars: histotroph; thick empty arrows: uterine epithelium; thick black arrows: trophoblast (TB). IHC. DAB. (Bar=50 μm).
3.2. Immunostained area
The percentage of immunostained area for IGF1 and IGF2 was higher in G1 than in G2 (p< 0.001 and p<= 0.002, respectively) (Figure 6A, B). In contrast, no differences in IGF1R immunostained area were observed between groups (p=0.1558) (Figure 6C).
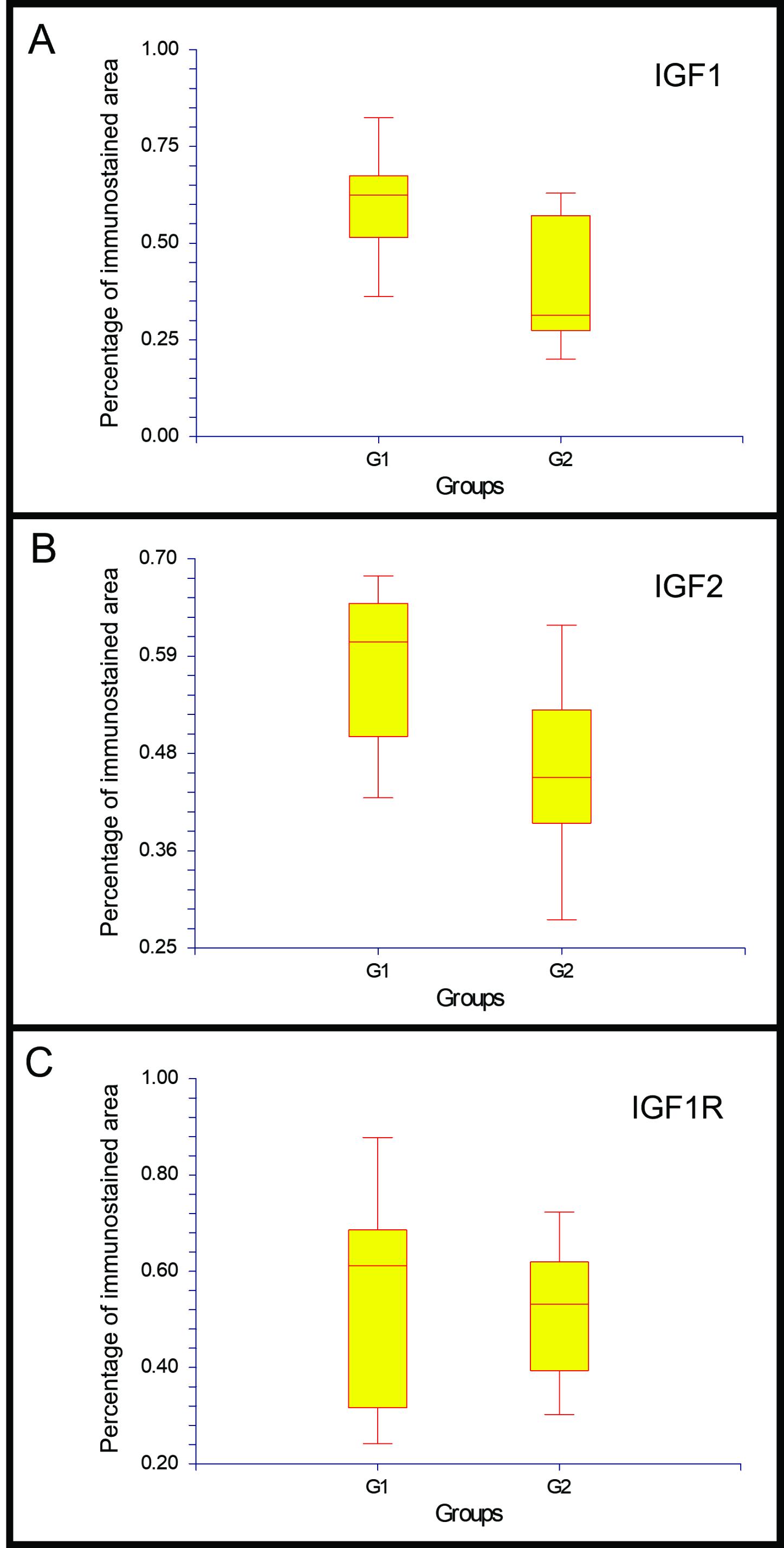
Figure 6
Percentage of the immunostained area in the placenta and gravid uterus of the cat. P values for the differences between groups 1 and 2 were: p= 0.00000001; n=10 for IGF1 (A), p= 0.0002; n=10 for IGF2 (B), and p=0.1558; n=10 for IGF1R (C).
4. Discussion and conclusions
We investigated the presence and particular localization of IGF1, IGF2, and IGF1R of different gestational age placentas and studied if there was a variation in their expression between placentas in G1 (≤43 d.p.c) and G2 (≥44 d.p.c). As it has been determined for other proteins in the cat placenta (Santos et al., 2021), for most of the structures studied here the qualitative analysis showed either a stronger immunohistochemical marking in early placentas than in later ones, or equal labelling in both groups. This was supported by results regarding the total percentage of immunolabeled area, which were consistently and significatively higher in early placentas for both IGF ligands. Stronger labelling in G1 might reflect the involvement of these molecules in signalling pathways required for early placental development (Guzeloglu-Kayisli et al., 2009; Sferruzzi-Perri et al., 2017). Our finding agrees with Freese et al. (2005) in sow gilts; they reported that glandular IGF1 expression was high during early gestation and decreased as the pregnancy progressed. Ağaoğlu et al. (2021) and ourselves (Hernández et al., 2020) observed a similar tendency in ≤ 45 d.p.c feline and from 28 to 62 d.p.c canine uteri, respectively. The similar pattern in uteri from these two species with endotheliochorial placentas strengthens the hypothesis that endometrial IGFs stimulate early placental development through histotrophic signalling, as also occurs in hemochorial placentas, where histotrophic mediated communication is crucial in early pregnancy (Wooding & Burton, 2008).
The IGF system is involved in the proliferation, growth, and differentiation of smooth myometrial myocytes in humans (Tang et al., 1994). Insulin-like growth factor 1 and 2 and IGF1R myometrial expression during pregnancy was also described in other species, such as pigs, rats, and dogs (Hernández et al., 2020; Huynh, 2000; Shynlova et al., 2007; Simmen et al., 1992). Results regarding myometrial staining at different stages of gestation are partially consistent with findings in other species. In rats, it was shown that while Igf2 and Igf1r remained virtually unchanged, Igf1 expression was higher in the myometrium during the first half of the gestation, concurring with the phase of increased myocyte proliferation (Shynlova et al., 2007). In dogs, IGF2 detection followed that tendency (Hernández et al., 2020), while IGF1 did not. Here, IGF1 and IGF2 remained relatively constant; IGF1R did not vary either, which agrees with results from other studies (Hernández et al., 2020; Shynlova et al., 2007).
The fact that JZ-CTB labelling intensity and intergroup variation have followed those of glands and histotroph might be related to the phagocytic activity of this type of CTB, which was mainly denuded, or at least split from JZ-STB. That activity may also account for JZ-CTB stronger labelling compared to labyrinthine CTB cells. This relation with the absorption of the histotroph may also explain the staining differences between JZ-CTB and JZ-STB. JZ-STB showed a mild and inconsistent reaction to the three antibodies; even being adjacent to the histotroph and the endometrial glands, this trophoblast cell type almost lacks phagocytic activity (Leiser & Koob, 1993).
Insulin-like growth factors exert several functions in trophoblastic cells. As was described above, IGFs/IGF1R expression has been studied mainly in the trophoblast of hemochorial placentas and, to a lesser extent, in epithelial and synepitheliochorial placentas. Among carnivores, the expression of these growth factors in invasive trophoblast was recently described in canine placentas (Hernández et al., 2020).
The stronger labelling of IGFs in CTB compared to that in STB may obey the central role that these growth factors play in CTB differentiation toward STB; IGFs enhance cytotrophoblast proliferation and syncytial formation and can rescue trophoblastic cells from apoptosis, acting via IGF1R (Forbes et al., 2008b). Syncytiotrophoblast formation depends on the continued proliferation and differentiation of CTB cells, as STB does not proliferate on its own (Forbes & Westwood, 2008a). In mice, signalling through IGF1R promotes STB formation and increases its activity. Expression of IGF1R in both CTB and STB in cats might be related, therefore, to placental development, growth and function, as it is also in women and guinea pigs (Martín-Estal et al., 2021). Whether the origin of syncytial IGFs is entirely cytotrophoblastic or they are partly synthesised in the STB is unknown. Currently, the occurrence of transcriptional and translational activities in the STB is a matter of controversy. While some authors argue that both processes remain active in the STB (Fogarty et al., 2011; Sferruzzi-Perri et al., 2011), others maintain that transcription and translation are downregulated in the syncytium (Ellery et al., 2009; Huppertz, 2010). In human placentas, transcription and translation of IGF1 in different trophoblastic populations and of IGF2 only in the extravillous trophoblast were recorded (Han & Carter, 2000; Hidden et al., 2009). Dubova et al. (2014) detected IGF1 and IGF2 both in the extravillous trophoblast and STB. Han & Carter et al.,(2000) reported IGF2 mRNA in the rhesus monkey in the same localisation. IGF2 was detected in several trophoblastic cells, e.g., those from the highly invasive human extravillous trophoblast or the glycogen cells in mice, which are both related to decidual invasion (Pollheimer et al., 2011; Roberts et al., 2008). Insulin-like growth factor 1 receptors were widely expressed in placental tissues in the first trimester and at term (Martín-Estal et al., 2021).
The distribution of IGFs in placentas of other domestic species has also been studied. In the mare, which develops an epitheliochorial placenta, IGF1 has been localised to the entire trophoblast in the microcotyledons (Arai et al., 2006); however, only the cells from the endometrial cups (the more invasive cell population) produce IGF2 (Lennard et al., 1995). In ewes, IGF2 transcription was restricted to maternal tissues and mesenchyme (Igwebuike, 2010). In invasive placentas, IGFs are initially secreted by the endometrium; then, as pregnancy progresses, both maternal and fetal placental cells produce IGFs (Bowman et al., 2010). In rhesus monkeys, it has been found a higher expression of IGF1 in phagocytic trophoblast facing the histotroph and a higher expression of IGF2 in invasive trophoblastic cells at the first stage of the placentation (Dhara et al., 2001). This contributes to the idea that different functions of IGF1 and IGF2 are evolutionarily conserved among species. Until now, we have not found any further information about the presence of either IGF proteins or transcripts in feline trophoblastic populations.
This different distribution of IGF2 among species and types of placentas might be linked to the function of IGF2 in cell migration and invasion achieved through IGF1R (Sferruzzi-Perri, 2018). Insulin-like growth factor 2 may stimulate invasion by regulating matrix metalloproteinase 2 and 9 availability, exerting a greater effect on these enzymes than IGF1 does (Hills et al., 2004). Those proteinases have been detected in endotheliochorial placentas (Diessler et al., 2017). In pigs around day 60 of gestation, IGF2 expression increases (Freese et al., 2005), coinciding with a time of marked fetal growth rise (Leiser & Koob, 1993). In baboons, IGF2 mRNA in the syncytiotrophoblast increases markedly as gestation advances (Zollers et al., 2001). Here, a corresponding change in IGF2 protein has not been detected. This may obey either interspecific differences or IGF2 mRNA´s lower translation rate.
The regulatory role of DCs on trophoblastic populations (Guzeloglu-Kayisli et al., 2009), mainly through IGFBP1, may account for the shortage of IGFs in the feline STB contiguous to DCs. Conversely, in the dog, a species with scarce DCs, the IGF signal in the STB is stronger than in cat samples.
Positivity for the three antibodies observed in the decidual cells agrees with that in bitches (Hernández, unpublished results). Insulin-like growth factor 1, 2, and 1 receptor RNA transcripts have been detected in canine stromal decidualized cells in culture (Kautz et al., 2015). Human DCs also express IGF1, IGF2, and IGF1R, as well as IGFBP1 and prolactin. The last two are regarded as decidual markers in this species and have also been detected in feline DCs (Hayati et al., 2007; Hernández et al., 2017, 2019; Hill et al., 1993). Decidual IGFBP1 is involved in IGFs regulation (Guzeloglu-Kayisli et al., 2009). Depending on IGFBP1 posttranslational modifications, mainly phosphorylation, it may have more or less affinity for IGFs. The unphosphorylated form shows more affinity for growth factors, leading to extravillous trophoblast migration; phosphorylated IGFBP1, on the contrary, has less affinity for growth factors (Forbes & Westwood, 2008a; Guzeloglu-Kayisli et al., 2009).
Reciprocal regulation between trophoblastic cells and DCs results in a timed invasion and normal placentation in humans (Hess et al., 2007). Based on our results and those in other species, it is reasonable to postulate that the DCs and the trophoblast at the cat maternal-fetal interface signal through IGFs and IGF1R, among other molecules, by auto and paracrine ways.
The involvement of the IGF system in angiogenesis is well-known. Endothelium responds to IGFs, released by other cells or synthesized locally, mainly by their binding to IGF1R. Signalling transduction downstream is key for vessel formation, as IGFs stimulate hypoxia-inducible factor-dependent and independent VEGF synthesis (Bach, 2015). In vitro studies showed that trophoblastic IGF1 stimulates proliferation and inhibits apoptosis in human placental endothelial cells (Troja et al., 2014). Furthermore, IGF2, which is considered a pregnancy-specific angiogenic factor, stimulates endothelial cell migration (Herr et al., 2003). IGF central participation in placental angiogenesis might account for the stronger labelling of maternal endothelia in the labyrinth than in the uterus itself.
In the free polar zone, the trophoblast is composed of vacuolated columnar cells, which phagocytose uterine secretions that keep it separate from the lining endometrial epithelium. This region resembles areolar zones in gilts and mares (Wooding & Burton, 2008). Positivity for IGFs and IGF1R found in trophoblastic cells may obey to phagocytosis of uterine milk rather than to their local expression.
Taken as a whole, our results indicate that IGFs and IGF1R are expressed in almost all the structures forming the placenta, both in early and late placentas, except for JZ-STB. It is a remarkable finding that IGF2 expression tended to be higher in early placentas, which could be due to its role in invasion. Regarding the maternal zone, IGF2 labelling was higher in the earliest samples than IGF1 and IGF1R.
These proteins have been studied in the queen pregnant uteri before, but only in maternal structures. As far as we know, this work constitutes the first one recording immunohistochemical IGFs/IGF1R detection in fetal regions of the placenta. Their high expression and wide distribution coincide with what has been found in another carnivore: the dog. These results may provide insight into the centrality of the IGF system during gestation and prenatal development in species with endotheliochorial placentas.
Financial support
This work was supported by the National University of La Plata (UNLP) [grant number: V229, V270]; and the Scientific and Technological Promotion National Agency (ANPCYT) [grant numbers PICT-2012-2649, PICT-2017-3530].
Author contributions
Rocío Hernández: conceptualization, formal analysis, investigation, methodology, validation, visualization, roles/writing - original draft. Gimena Gomez Castro: investigation, formal analysis, validation, visualization. Fernanda M. Rodriguez: investigation, validation, visualization. Luciano Casas: investigation, visualization. Enrique Portiansky: formal analysis, writing - review & editing. Claudio Barbeito: conceptualization, funding acquisition, resources, project administration, supervision, writing - review & editing. Florencia Rey: funding acquisition, resources, supervision, writing - review & editing. Mónica E. Diessler: conceptualization, methodology, supervision, funding acquisition, writing - review & editing.
Declarations of interest: None, the authors declare that they have no competing interests.
Acknowledgments
The authors thank MV J. Herrera for his collaboration in surgical procedures and the ICiVet-Litoral lab staff (especially Dr. N. Gareis).
References
Ağaoğlu OK, Ağaoğlu AR, Guzeloglu A, Aslan S, Kurar E, Kayis SA, Schäfer-Somi S. 2016. Gene expression profiles of some cytokines, growth factors, receptors, and enzymes (GM-CSF, IFNγ, MMP‑2, IGF-II, EGF, TGF-β, IGF-IIR) during pregnancy in the cat uterus. Theriogenology. 85(4):638-44. http://dx.doi.org/10.1016/j.theriogenology.2015.10.001
Ağaoğlu OK, Ağaoğlu AR, Özmen Ö, Saatci M, Schäfer-Somi S, Aslan S. 2021. Expression of the insulin-like growth factors (IGF) gene family in feline uterus during pregnancy. Biotechnic & Histochemistry. 96(6):439–49. http://dx.doi.org/10.1080/10520295.2020.1818285
Arai KY, Tanaka Y, Taniyama H, Tsunoda N, Nambo Y, Nagamine N, Watanabe G, Taya K. 2006. Expression of inhibins, activins, insulin-like growth factor-I and steroidogenic enzymes in the equine placenta. Domestic Animal Endocrinology. 31(1):19-34. https://doi.org/10.1016/j.domaniend.2005.09.005
Bach LA. 2015. Endothelial cells and the IGF system. Journal of Molecular Endocrinology. 54(1):R1-R13. http://dx.doi.org/10.1530/JME-14-0215
Baravalle ME, Stassi AF, Velázquez MML, Belotti EM, Rodríguez FM, Ortega HH, Salvetti NR. 2015. Altered expression of pro-inflammatory cytokines in ovarian follicles of cows with cystic ovarian disease. Journal of Comparative Pathology. 153(2-3):116-30. http://dx.doi.org/10.1016/j.jcpa.2015.04.007
Boomsma RA, Mavrogianis PA, Fazleabas AT, Jaffe RC, Verhage HG. 1994. Detection of insulin-like growth factor binding protein-1 in cat implantation sites. Biology of Reproduction. 51(3):392-99. http://dx.doi.org/10.1095/biolreprod51.3.392
Bowman CJ, Streck RD, Chapin RE. 2010. Maternal-placental insulin-like growth factor (IGF) signaling and its importance to normal embryo-fetal development. Birth Defects Research. Part B, Developmental and Reproductive Toxicology. 89(4):339-49. http://dx.doi.org/10.1002/bdrb.20249
Cherif-Feildel M, Berthelin CH, Rivière G, Favrel P, Kellner K. 2019. Data for evolutive analysis of insulin related peptides in bilaterian species. Data in Brief. 22:546-50. http://dx.doi.org/10.1016/j.dib.2018.12.050
Dhara S, Lalitkumar PGL, Sengupta J, Ghosh D. 2001. Immunohistochemical localization of insulin-like growth factors I and II at the primary implantation site in the Rhesus monkey. Molecular Human Reproduction. 7(4):365-71. http://dx.doi.org/10.1093/molehr/7.4.365
Diessler M, Ventureira M, Hernández R, Sobarzo C, Casas L, Barbeito C, Cebral E. 2017. Differential expression and activity of matrix metalloprotease-2 and -9 in canine early placenta. Reproduction in Domestic Animals. 52(1):35-43. http://dx.doi.org/10.1111/rda.12791
Diessler ME, Idiart JR, Portiansky EL. 2007. Metástasis y angiogénesis en carcinomas mamarios invasivos de perras diagnosticados entre 1980 y 2003. Estudios histológicos e inmunohistoquímicos. Analecta Veterinaria. 27(1):5-10. http://sedici.unlp.edu.ar/handle/10915/11197
Dubova EA, Pavlov KA, Lyapin VM, Kulikova GV, Shchyogolev AI, Sukhikh GT. 2014. Expression of insulin-like growth factors in the placenta in pre-eclampsia. Bulletin of Experimental Biology and Medicine. 157(1):103-107. http://dx.doi.org/10.1007/s10517-014-2502-4
Ellery PM, Cindrova-Davies T, Jauniaux E, Ferguson-Smith AC, Burton GJ. 2009. Evidence for transcriptional activity in the syncytiotrophoblast of the human placenta. Placenta. 30(4):329-34. http://dx.doi.org/10.1016/j.placenta.2009.01.002
Evans HE, Sack WO. 1973. Prenatal development of domestic and laboratory mammals: growth curves, external features and selected references. Zentralblatt fur Veterinarmedizin. Reihe C: Anatomie, Histologie, Embryologie. 2(1):11-45. http://dx.doi.org/10.1111/j.1439-0264.1973.tb00253.x
Fogarty NME, Mayhew TM, Ferguson-Smith AC, Burton GJ. 2011. A quantitative analysis of transcriptionally active syncytiotrophoblast nuclei across human gestation. Journal of Anatomy. 219(5):601-10. http://dx.doi.org/10.1111/j.1469-7580.2011.01417.x
Forbes K, Westwood M. 2008a. The IGF axis and placental function: a mini review. Hormone Research. 69(3):129-37.
Forbes K, Westwood M, Baker PN, Aplin JD. 2008b. Insulin-like growth factor I and II regulate the life cycle of trophoblast in the developing human placenta. American Journal of Physiology-Cell Physiology. 294(6):C1313-22. http://dx.doi.org/10.1152/ajpcell.00035.2008
Freese LG, Rehfeldt C, Fuerbass R, Kuhn G, Okamura CS, Ender K, Grant AL, Gerrard DE. 2005. Exogenous somatotropin alters IGF axis in porcine endometrium and placenta. Domestic Animal Endocrinology. 29(3):457-75. http://dx.doi.org/10.1016/j.domaniend.2005.02.012
Guzeloglu-Kayisli O, Kayisli UA, Taylor HS. 2009. The role of growth factors and cytokines during implantation: endocrine and paracrine interactions. Seminars in Reproductive Medicine. 27(1):62-79. http://dx.doi.org/10.1055/s-0028-1108011
Han VKM, Carter AM. 2000. Spatial and temporal patterns of expression of messenger RNA for insulin-like growth factors and their binding proteins in the placenta of man and laboratory animals. Placenta. 21(4):289-305. http://dx.doi.org/10.1053/plac.1999.0498
Harris LK, Pantham P, Yong HEJ, Pratt A, Borg AJ, Crocker I, Westwood M, Aplin J, Kalionis B, Murthi P. 2019. The role of insulin-like growth factor 2 receptor-mediated homeobox gene expression in human placental apoptosis, and its implications in idiopathic fetal growth restriction. Molecular Human Reproduction. 25(9):572-85. http://dx.doi.org/10.1093/molehr/gaz047
Hayati AR, Cheah FC, Tan AE, Tan GC. 2007.Insulin-like growth factor-1 receptor expression in the placentae of diabetic and normal pregnancies. Early Human Development. 83(1):41-6. http://dx.doi.org/10.1016/j.earlhumdev.2006.04.002
Hernández R, Barbeito C, Diessler M. 2019. Immunohistochemical detection of prolactin and prolactin receptor in canine and feline placentae. Placenta.83:e71-e73. https://doi.org/10.1016/j.placenta.2019.06.229
Hernández R, Gareis N, Stassi A, Blois SM, Fernández P, Zanuzzi C, Rey F, Barbeito CG, Diessler M. 2017. Insulin-like growth factor binding protein-1, galectin-9, vimentin, desmin and α-actin expression in decidualized stromal cells from canine and feline placental labyrinth. Placenta. 51:122. http://dx.doi.org/10.1016/j.placenta.2017.01.082
Hernández R, Rodríguez FM, Gareis NC, Rey F, Barbeito CG, Diessler M. 2020. Abundance of insulin-like growth factors 1 and 2, and type 1 insulin-like growth factor receptor in placentas of dogs. Animal Reproduction Science. 221:106554. http://dx.doi.org/10.1016/j.anireprosci.2020.106554
Herr F, Baal N, Zygmunt M. 2009. Studies of placental vasculogenesis: a way to understand pregnancy pathology? Zeitschrift für Geburtshilfe und Neonatologie. 213(3):96-100. http://dx.doi.org/10.1055/s-0029-1224141
Herr F, Liang OD, Herrero J, Lang U, Preissner KT, Han VKM., Zygmunt M. 2003. Possible angiogenic roles of insulin-like growth factor II and its receptors in uterine vascular adaptation to pregnancy. The Journal of Clinical Endocrinology & Metabolism. 88(10):4811-7. http://dx.doi.org/10.1210/jc.2003-030243
Hess AP, Hamilton AE, Talbi S, Dosiou C, Nyegaard M, Nayak N, Genbecev-Krtolica O, Mavrogianis P, Ferrer K, Kruessel J, Fazleabas AT, Fisher SJ, Giudice LC. 2007. Decidual stromal cell response to paracrine signals from the trophoblast: amplification of immune and angiogenic modulators. Biology of Reproduction. 76(1):102-17. http://dx.doi.org/10.1095/biolreprod.106.054791
Hidden U, Glitzner E, Harttmann M, Desoye G. 2009. Insulin and the IGF system in the human placenta of normal and diabetic pregnancies. Journal of Anatomy. 215(1):60-8. http://dx.doi.org/10.1111/j.1469-7580.2008.01035.x
Hill DJ, Clemmons DR, Riley SC, Bassett N, Challis JRG. 1993. Immunohistochemical localization of insulin-like growth factors (IGFs) and IGF binding proteins--1, -2 and -3 in human placenta and fetal membranes. Placenta. 14(1):1-12. http://dx.doi.org/10.1016/s0143-4004(05)80244-9
Hills FA, Elder MG, Chard T, Sullivan MHF. 2004. Regulation of human villous trophoblast by insulin-like growth factors and insulin-like growth factor-binding protein-1. Journal of Endocrinology. 183(3):487-96. http://dx.doi.org/10.1677/joe.1.05867
Huppertz B. 2010. IFPA Award in Placentology Lecture: biology of the placental syncytiotrophoblast – myths and facts. Placenta. 31:S75–S81. http://dx.doi.org/10.1016/j.placenta.2009.12.001
Huynh H. 2000. Posttranscriptional and posttranslational regulation of insulin-like growth factor binding protein-3 and -4 by insulin-like growth factor-I in uterine myometrial cells. Growth Hormone & IGF Research. 10(1):20-7. http://dx.doi.org/10.1054/ghir.2000.0137
Igwebuike UM. 2010. Impact of maternal nutrition on ovine foetoplacental development: a review of the role of insulin-like growth factors. Animal Reproduction Science. 121(3-4):189-96. http://dx.doi.org/10.1016/j.anireprosci.2010.04.007
Kautz E, de Carvalho Papa P, Reichler IM, Gram A, Boos A, Kowalewski MP. 2015. In vitro decidualisation of canine uterine stromal cells. Reproductive Biology and Endocrinology. 13-85. http://dx.doi.org/10.1186/s12958-015-0066-4
Kautz E, Gram A, Aslan S, Ay SS, Selçuk M, Kanca H, Koldaş E, Akal E, Karakaş K, Findik M, Boos A, Kowalewski MP. 2014. Expression of genes involved in the embryo-maternal interaction in the early-pregnant canine uterus. Reproduction. 147(5):703-17. http://dx.doi.org/10.1530/REP-13-0648
Knospe C. 2002. Periods and stages of the prenatal development of the domestic cat. Anatomia, Histology, Embryology. 31(1):37-51. http://dx.doi.org/10.1046/j.1439-0264.2002.00360.x
Leiser R, Koob B. 1993. Development and characteristics of placentation in a carnivore, the domestic cat. Journal of Experimental Zoology. 266(6):642-56. http://dx.doi.org/10.1002/jez.1402660612
Lennard SN, Stewart F, Allen WR. 1995. Insulin-like growth factor II gene expression in the fetus and placenta of the horse during the first half of gestation. Journal of Reproduction and Fertility. 103(1):169-79. http://dx.doi.org/10.1530/jrf.0.1030169
LeRoith D, Holly JMP, Forbes BE. 2021. Insulin-like growth factors: Ligands, binding proteins, and receptors. Molecular Metabolism. 52:101245. http://dx.doi.org/10.1016/j.molmet.2021.101245
Liu X, Hu Q, Liu S, Tallo LJ, Sadzewicz L, Schettine CA, Nikiforov M, Klyushnenkova EN, Ionov Y. 2013. Serum antibody repertoire profiling using in silico antigen screen. PLoS One. 8(6):e67181. http://dx.doi.org/10.1371/journal.pone.0067181
Martín-Estal I, Castilla-Cortázar I, Castorena-Torres F. 2021. The placenta as a target for alcohol during pregnancy: the close relation with IGFs signaling pathway. Reviews of Physiology, Biochemistry and Pharmacology. 180:119-53. http://dx.doi.org/10.1007/112_2021_58
Pieri N, Souza AF, Casals JB, Roballo K, Ambrosio CE, Martins DS. 2015. Comparative development of embryonic age by organogenesis in domestic dogs and cats. Reproduction in Domestic Animals. 50(4):625-31. http://dx.doi.org/10.1111/rda.12539
Pollheimer J, Haslinger P, Fock V, Prast J, Saleh L, Biadasiewicz K, Jetne-Edelmann R, Haraldsen G, Haider S, Hirtenlehner-Ferber K, Knöfler M. 2011. Endostatin suppresses IGF-II-mediated signaling and invasion of human extravillous trophoblasts. Endocrinology. 152(11):4431-42. http://dx.doi.org/10.1210/en.2011-1196
Rentería ME, Gandhi NS, Vinuesa P, Helmerhorst E, Mancera RL. 2008. A comparative structural bioinformatics analysis of the insulin receptor family ectodomain based on phylogenetic information. PLoS One. 3(11):e3667. http://dx.doi.org/10.1371/journal.pone.0003667
Roberts CT, Owens JA, Sferruzzi-Perri AN. 2008. Distinct actions of insulin-like growth factors (IGFs) on placental development and fetal growth: lessons from mice and guinea pigs. Placenta. 29(Suppl. A):S42-S47. http://dx.doi.org/10.1016/j.placenta.2007.12.002
Roncador G, Engel P, Maestre L, Anderson AP, Cordell JL, Cragg MS, Šerbec VK, Jones M, Lisnic VJ, Kremer L, Li D, Koch-Nolte F, Pascual N, Rodríguez-Barbosa JI, Torensma R, Turley H, Pulford K, Banham AH. 2015. The European antibody network’s practical guide to finding and validating suitable antibodies for research. mAabs. 8(1):27-36. http://dx.doi.org/10.1080/19420862.2015.1100787
Santos LC, Dos Anjos Cordeiro JM, da Silva Santana L, Santos BR, Barbosa EM, da Silva TQM, Corrêa JMX, Niella RV, Lavor MSL, da Silva EB, de Melo Ocarino N, Serakides R, Silva JF. 2021. Kisspeptin/Kiss1r system and angiogenic and immunological mediators at the maternal-fetal interface of domestic cats. Biology of Reproduction. 105(1):217-31. http://dx.doi.org/10.1093/biolre/ioab061
Sferruzzi-Perri AN, Owens JA, Pringle KG, Roberts CT. 2011. The neglected role of insulin-like growth factors in the maternal circulation regulating fetal growth. The Journal of Physiology. 589(1):7-20. http://dx.doi.org/10.1113/jphysiol.2010.198622
Sferruzzi-Perri AN, Sandovici I, Constancia M, Fowden AL. 2017. Placental phenotype and the insulin-like growth factors: resource allocation to fetal growth. The Journal of Physiology. 595:5057-93. http://dx.doi.org/10.1113/JP273330
Sferruzzi-Perri AN. 2018. Regulating needs: exploring the role of insulin-like growth factor-2 signalling in materno-fetal resource allocation. Placenta. 64(1):S16-S22. http://dx.doi.org/ 10.1016/j.placenta.2018.01.005
Sharma S, Godbole G, Modi D. 2016. Decidual control of trophoblast invasion. American Journal of Reproductive Immunology. 75(3):341-50. http://dx.doi.org/10.1111/aji.12466
Shynlova O, Tsui P, Dorogin A, Lowell Langille B, Lye SL. 2007. Insulin-like growth factors and their binding proteins define specific phases of myometrial differentiation during pregnancy in the rat. Biology of Reproduction. 76(4):571-8. http://dx.doi.org/10.1095/biolreprod.106.056929
Shynlova O, Tsui P, Jaffer S, Lye SL. 2009. Integration of endocrine and mechanical signals in the regulation of myometrial functions during pregnancy and labor. European Journal of Obstetrics & Gynecology and Reproductive Biology. 144(1):S2-S10. http://dx.doi.org/10.1016/j.ejogrb.2009.02.044
Simmen FA, Simmen RC, Geisert RD, Martinat-Botte F, Bazer FW, Terqui M. 1992. Differential expression, during the estrous cycle and pre- and post- implantation conceptus development, of messenger ribonucleic acids encoding components of the pig uterine insulin-like growth factor system. Endocrinology. 130(3):1547-56. http://dx.doi.org/10.1210/endo.130.3.1537304
Stassi AF, Gasser F, Velázquez MML, Belotti EM, Gareis NC, Rey F, Ortega HH, Salvetti NR, Baravalle ME. 2019. Contribution of the VEGF system to the follicular persistence associated with bovine cystic ovaries. Theriogenology. 138:52-65. http://dx.doi.org/10.1016/j.theriogenology.2019.07.002
Tang XM, Rossi MJ, Masterson BJ, Chegini N. 1994. Insulin-like growth factor I (IGF-I), IGF-I receptors, and IGF binding proteins 1-4 in human uterine tissue: tissue localization and IGF-I action in endometrial stromal and myometrial smooth muscle cells in vitro. Biology of Reproduction. 50(5):1113-25. http://dx.doi.org/10.1095/biolreprod50.5.1113
Troja W, Kil K, Klanke C, Jones HN. 2014. Interaction between human placental microvascular endothelial cells and a model of human trophoblasts: effects on growth cycle and angiogenic profile. Physiological Reports. 2(3):e00244. http://dx.doi.org/10.1002/phy2.244
Wooding FB, Stewart F, Mathias S, Allen WR. 2005. Placentation in the African elephant, Loxodonta africanus: III. Ultrastructural and functional features of the placenta. Placenta. 26(6):449-70. http://dx.doi.org/10.1016/j.placenta.2004.08.007
Wooding P, Burton G. 2008. Comparative placentation: structures, functions, and evolution. Springer Berlin, Heidelberg. https://doi.org/10.1007/978-3-540-78797-6
Zollers Jr. WG, Babischkin JS, Pepe GJ, Albrecht ED. 2001. Developmental regulation of placental insulin-like growth factor (IGF)-II and IGF-binding protein-1 and -2 messenger RNA expression during primate pregnancy. Biology of Reproduction. 65(4):1208-14. http://dx.doi.org/10.1095/biolreprod65.4.1208
Author notes
Correo electrónico de contacto del autor correspondiente: rhernandez@fcv.unlp.edu.ar


