Development a three-dimensional model of a murine mammary adenocarcinoma from a monolayer culture
Keywords:
mammospheres, murine mammary adenocarcinoma, mesenchymal stem cellsAbstract
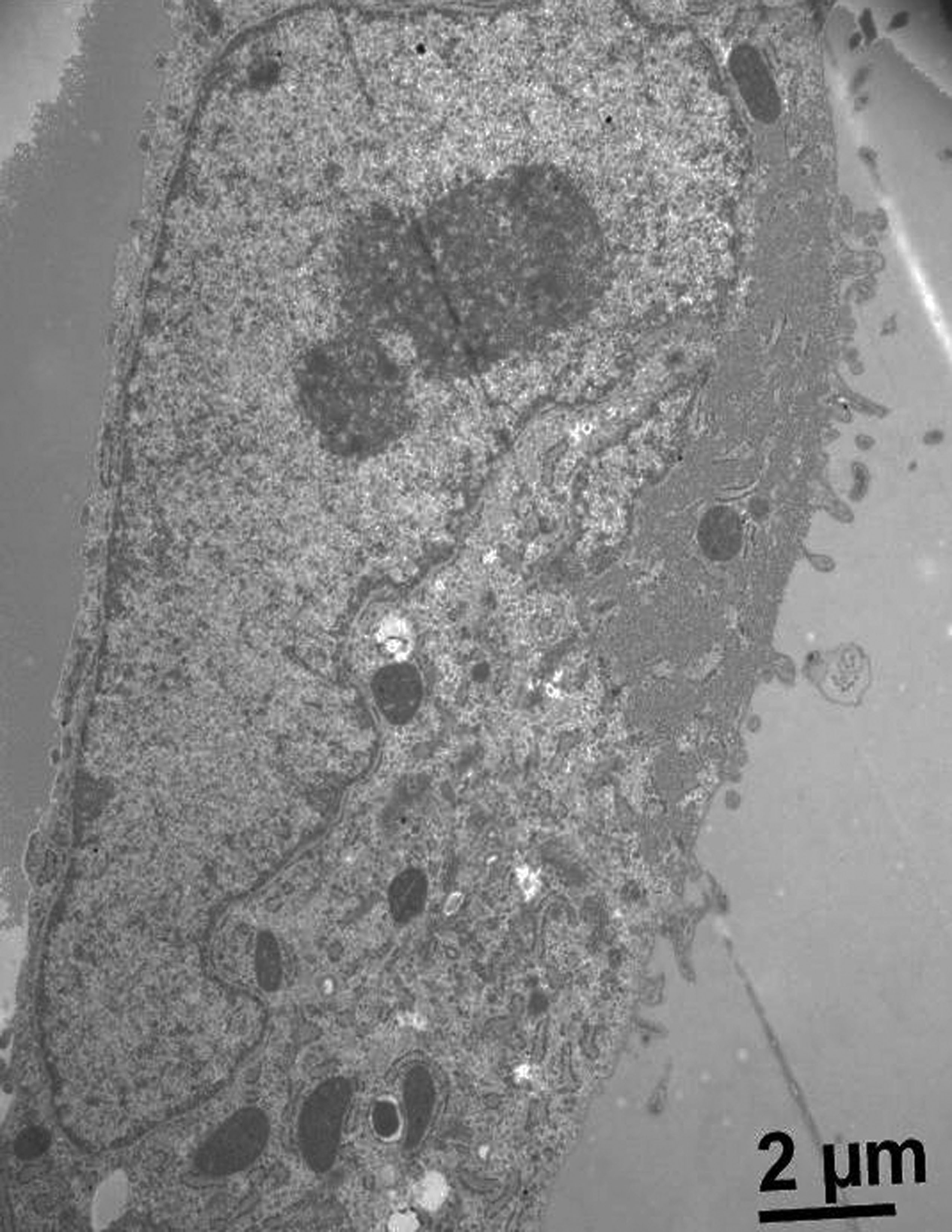
The mammospheres are a multicellular tumor spheroid model. These are a widely used culture system to mimic the three-dimensionality of tumors. In this study, we develop this model using a murine mammary adenocarcinoma, which is used to study tumor physiology and therapeutic applications. We assay some variants of liquid overlay technique to know the best technique to generate this mammospheres. The best condition was used V-shaped 96-wells plates with an agarose monolayer because we were obtained compact and robust spheroids. Additionally, for evaluate spheroid´s structure by optical microscopy, we performed histologic staining of the mammospheres with hematoxylin-eosin. Moreover, we made immunohistochemistry staining to evaluate cell proliferation index and neovascularization, with bromodeoxyuridine and VEGF (vascular endothelial growth factor), respectively. On the other hand, we studied the interaction between tumoral cells and mesenchymal stem cells derived to human umbilical cord.
Downloads
References
Clarke KE, Tams DM, Henderson AP, Roger MF, Whiting A, Przyborski SA- (2016). A robust and reproducible human pluripotent stem cell derived model of neurite outgrowth in a three-dimensional culture system and its application to study neurite inhibition. Neurochem Int, 106:74-84.
Costa EC, Gaspar VM, Coutinho P, Correia IJ- (2014). Optimization of Liquid Overlay Technique to Formulate Heterogenic 3D Co-Cultures Models. Biotechnol. Bioeng. 11:1672-1685.
Cuiffo BG and Karnoub A- (2012). Mesenchymal stem cells in tumor development. Emerging roles and concepts. Cell Adh Migr, 6:220-230.
Fleisig H, Wong J- (2012). Measuring Cell Cycle Progression Kinetics with Metabolic Labeling and Flow Cytometry. J. Vis. Exp22:1-6.
Furnus CC, Inda AM, Andrini LB, García MN, García AL, Badrán A, Errecalde AL- (2003). Chronobiology of the proliferative events related to angiogenesis in mice liver regeneration after partial hepatectomy. Cell Biol Int 27: 383–386.García MN, Andrini LB, Inda AM, Ronderos JR, Hijano JC, Errecalde A- (2010). Changes in VEGF expression and DNA synthesis in hepatocytes from hepatectomized and tumour-bearing mice. Cell Biol. Int. 34:283–286.
García MN, Andrini L, Martinez M, Inda A, Palma MB, Miriuka S, Errecalde AL- (2015). Circadian rhythms of proliferation events in two mouse carcinomas. Biol- Rhythm Res. 46:573-578.
Hasselbach LA, Irtenkauf SM, Lemke NW, Nelson KK, Berezovsky AD, Carlton ET, Transou AD, Mikkelsen T, deCarvalho A- (2014). Optimization of High Grade Glioma Cell Culture from Surgical Specimens for Use in Clinically Relevant Animal Models and 3D Immunochemistry. J. Vis. Exp. 83:1-9.
Inda AM, García MN, Andrini LB, García AL, Fernández Blanco A, Furnus CC, Galletti SM, Brandoni J, Martinez JG, Prat GD, Errecalde AL- (2009). Evaluation of Angiogenesis with the Expression of VEGF-C and CD34 in Human Colon Cancer. Current Chemical Biology 3: 302-305.
Lee G, Hall R, Ahmed A- (2016). Cancer stem cells: celular plasticity, niche and its clinical relevance. J Stem Cell Res Ther. 6:1-20
Nagelkerke A, Bussink J, Sweep F, Span PN- (2013). Generation of multicellular tumor spheroids of breast cancer cells: How to go threedimensional. Anal Biochem 437: 17-19.
Razian G, Yu Y, Ungrin M- (2013). Production of large numbers of size-controlled tumor spheroids using microwell plates. J Vis Exp 81:1-6.
Solomon MA, Lemera J, D´Souza GG- (2016). Development of an in vitro tumor spheroid culture model amenable to high-throughput testing of potential anticancer nanotherapeutics. J Liposome Res. 26: 246-260.
Sun Z, Wang S, Chunhua Zhao R- (2014). The roles of mesenchymal stem cells in tumor inflamatory microenvironment. J Hematol Oncol. 7:2-10.
Suzuki K, Sun R, Origuchi M, Kanehira M, Takahata T, Itoh J, Umezawa A, Kijima H, Fukuda S, Saijo Y- (2011). Mesenchymal stromal cells promote tumor growth. Through the enhancement of neovascularization. Molmed. 17: 579-587.
Wharton´s jelly-derived multipotent mesenchymal stromal cells obtained from bovine umbilical cord and maintained in a defined serum-free three-dimensional system. BMC Biotechnology 12:1-11.
Wong C, Vosburgh E, Levine AJ, Cong L, Xu EY- (2012). Human neuroendocrine tumor cell lines as a three-dimensional model for the study of human neuroendocrine tumor therapy. J Vis Exp 66:1-7.
Downloads
Published
How to Cite
Issue
Section
License
1. Política propuesta para revistas de acceso abierto
Los autores/as que publiquen en esta revista aceptan las siguientes condiciones:
- Los autores/as conservan los derechos de autor y ceden a la revista el derecho de la primera publicación, con el trabajo registrado con la licencia de atribución de Creative Commons, que permite a terceros utilizar lo publicado siempre que mencionen la autoría del trabajo y a la primera publicación en esta revista.
- Los autores/as pueden realizar otros acuerdos contractuales independientes y adicionales para la distribución no exclusiva de la versión del artículo publicado en esta revista (p. ej., incluirlo en un repositorio institucional o publicarlo en un libro) siempre que indiquen claramente que el trabajo se publicó por primera vez en esta revista.
- Se permite y recomienda a los autores/as a publicar su trabajo en Internet (por ejemplo en páginas institucionales o personales) despues del proceso de revisión y publicación, ya que puede conducir a intercambios productivos y a una mayor y más rápida difusión del trabajo publicado.